Nomogram to predict rectal toxicity following prostate cancer radiotherapy
- PMID: 28640871
- PMCID: PMC5480987
- DOI: 10.1371/journal.pone.0179845
Nomogram to predict rectal toxicity following prostate cancer radiotherapy
Abstract
Background: To identify predictors of acute and late rectal toxicity following prostate cancer radiotherapy (RT), while integrating the potential impact of RT technique, dose escalation, and moderate hypofractionation, thus enabling us to generate a nomogram for individual prediction.
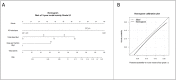
Methods: In total, 972 patients underwent RT for localized prostate cancer, to a total dose of 70 Gy or 80 Gy, using two different fractionations (2 Gy or 2.5 Gy/day), by means of several RT techniques (3D conformal RT [3DCRT], intensity-modulated RT [IMRT], or image-guided RT [IGRT]). Multivariate analyses were performed to identify predictors of acute and late rectal toxicity. A nomogram was generated based on the logistic regression model used to predict the 3-year rectal toxicity risk, with its accuracy assessed by dividing the cohort into training and validation subgroups.
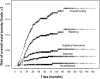
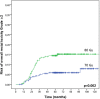
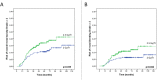
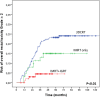
Results: Mean follow-up for the entire cohort was 62 months, ranging from 6 to 235. The rate of acute Grade ≥2 rectal toxicity was 22.2%, decreasing when combining IMRT and IGRT, compared to 3DCRT (RR = 0.4, 95%CI: 0.3-0.6, p<0.01). The 5-year Grade ≥2 risks for rectal bleeding, urgency/tenesmus, diarrhea, and fecal incontinence were 9.9%, 4.5%, 2.8%, and 0.4%, respectively. The 3-year Grade ≥2 risk for overall rectal toxicity increased with total dose (p<0.01, RR = 1.1, 95%CI: 1.0-1.1) and dose per fraction (2Gy vs. 2.5Gy) (p = 0.03, RR = 3.3, 95%CI: 1.1-10.0), and decreased when combining IMRT and IGRT (RR = 0.50, 95% CI: 0.3-0.8, p<0.01). Based on these three parameters, a nomogram was generated.
Conclusions: Dose escalation and moderate hypofractionation increase late rectal toxicity. IMRT combined with IGRT markedly decreases acute and late rectal toxicity. Performing combined IMRT and IGRT can thus be envisaged for dose escalation and moderate hypofractionation. Our nomogram predicts the 3-year rectal toxicity risk by integrating total dose, fraction dose, and RT technique.
Conflict of interest statement
Figures
References
-
- Gami B, Harrington K, Blake P, Dearnaley D, Tait D, Davies J, et al. How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther. 2003;18: 987–994. doi: 10.1046/j.1365-2036.2003.01760.x - DOI - PubMed
-
- Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-Term Results of the M. D. Anderson Randomized Dose-Escalation Trial for Prostate Cancer. Int J Radiat Oncol. 2008;70: 67–74. doi: 10.1016/j.ijrobp.2007.06.054 - DOI - PubMed
-
- Peeters STH, Heemsbergen WD, van Putten WLJ, Slot A, Tabak H, Mens JW, et al. Acute and late complications after radiotherapy for prostate cancer: Results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol. 2005;61: 1019–1034. doi: 10.1016/j.ijrobp.2004.07.715 - DOI - PubMed
-
- Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8: 475–487. doi: 10.1016/S1470-2045(07)70143-2 - DOI - PubMed
-
- Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, Clark C, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13: 43–54. doi: 10.1016/S1470-2045(11)70293-5 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

