NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato
- PMID: 28640975
- PMCID: PMC5787828
- DOI: 10.1111/pbi.12776
NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato
Abstract
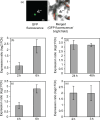
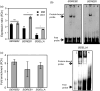
Water deficit (drought stress) massively restricts plant growth and the yield of crops; reducing the deleterious effects of drought is therefore of high agricultural relevance. Drought triggers diverse cellular processes including the inhibition of photosynthesis, the accumulation of cell-damaging reactive oxygen species and gene expression reprogramming, besides others. Transcription factors (TF) are central regulators of transcriptional reprogramming and expression of many TF genes is affected by drought, including members of the NAC family. Here, we identify the NAC factor JUNGBRUNNEN1 (JUB1) as a regulator of drought tolerance in tomato (Solanum lycopersicum). Expression of tomato JUB1 (SlJUB1) is enhanced by various abiotic stresses, including drought. Inhibiting SlJUB1 by virus-induced gene silencing drastically lowers drought tolerance concomitant with an increase in ion leakage, an elevation of hydrogen peroxide (H2 O2 ) levels and a decrease in the expression of various drought-responsive genes. In contrast, overexpression of AtJUB1 from Arabidopsis thaliana increases drought tolerance in tomato, alongside with a higher relative leaf water content during drought and reduced H2 O2 levels. AtJUB1 was previously shown to stimulate expression of DREB2A, a TF involved in drought responses, and of the DELLA genes GAI and RGL1. We show here that SlJUB1 similarly controls the expression of the tomato orthologs SlDREB1, SlDREB2 and SlDELLA. Furthermore, AtJUB1 directly binds to the promoters of SlDREB1, SlDREB2 and SlDELLA in tomato. Our study highlights JUB1 as a transcriptional regulator of drought tolerance and suggests considerable conservation of the abiotic stress-related gene regulatory networks controlled by this NAC factor between Arabidopsis and tomato.
Keywords: Arabidopsis; DELLA; drought; reactive oxygen species; tomato; transcription factor.
© 2017 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Overexpression of the NAC transcription factor JUNGBRUNNEN1 (JUB1) increases salinity tolerance in tomato.Plant Physiol Biochem. 2019 Jul;140:113-121. doi: 10.1016/j.plaphy.2019.04.038. Epub 2019 May 1. Plant Physiol Biochem. 2019. PMID: 31100704
-
The abiotic stress-responsive NAC-type transcription factor SlNAC4 regulates salt and drought tolerance and stress-related genes in tomato (Solanum lycopersicum).Plant Cell Rep. 2014 Nov;33(11):1851-63. doi: 10.1007/s00299-014-1662-z. Epub 2014 Jul 26. Plant Cell Rep. 2014. PMID: 25063324
-
SlDREB2, a tomato dehydration-responsive element-binding 2 transcription factor, mediates salt stress tolerance in tomato and Arabidopsis.Plant Cell Environ. 2016 Jan;39(1):62-79. doi: 10.1111/pce.12591. Epub 2015 Aug 8. Plant Cell Environ. 2016. PMID: 26082265
-
Characteristics of NAC transcription factors in Solanaceae crops and their roles in responding to abiotic and biotic stresses.Biochem Biophys Res Commun. 2024 May 21;709:149840. doi: 10.1016/j.bbrc.2024.149840. Epub 2024 Mar 28. Biochem Biophys Res Commun. 2024. PMID: 38564941 Review.
-
Phenotyping in Arabidopsis and Crops-Are We Addressing the Same Traits? A Case Study in Tomato.Genes (Basel). 2020 Aug 27;11(9):1011. doi: 10.3390/genes11091011. Genes (Basel). 2020. PMID: 32867311 Free PMC article. Review.
Cited by
-
HEBE, a novel positive regulator of senescence in Solanum lycopersicum.Sci Rep. 2020 Jul 3;10(1):11021. doi: 10.1038/s41598-020-67937-z. Sci Rep. 2020. PMID: 32620827 Free PMC article.
-
Overexpression of SlGATA17 Promotes Drought Tolerance in Transgenic Tomato Plants by Enhancing Activation of the Phenylpropanoid Biosynthetic Pathway.Front Plant Sci. 2021 Mar 16;12:634888. doi: 10.3389/fpls.2021.634888. eCollection 2021. Front Plant Sci. 2021. PMID: 33796125 Free PMC article.
-
Necrotic upper tips1 mimics heat and drought stress and encodes a protoxylem-specific transcription factor in maize.Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20908-20919. doi: 10.1073/pnas.2005014117. Epub 2020 Aug 10. Proc Natl Acad Sci U S A. 2020. PMID: 32778598 Free PMC article.
-
Integrative Analysis of Transcriptome and Metabolome Reveals the Pivotal Role of the NAM Family Genes in Oncidium hybridum Lodd. Pseudobulb Growth.Int J Mol Sci. 2024 Sep 26;25(19):10355. doi: 10.3390/ijms251910355. Int J Mol Sci. 2024. PMID: 39408686 Free PMC article.
-
Defense Strategies: The Role of Transcription Factors in Tomato-Pathogen Interaction.Biology (Basel). 2022 Feb 1;11(2):235. doi: 10.3390/biology11020235. Biology (Basel). 2022. PMID: 35205101 Free PMC article. Review.
References
-
- Achard, P. , Renou, J.P. , Berthomé, R. , Harberd, N.P. and Genschik, P. (2008b) Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 18, 656–660. - PubMed
-
- Al Abdallat, A.M. , Ayad, J.Y. , Abu Elenein, J.M. , Al Ajlouni, Z. and Harwood, W.A. (2014) Overexpression of the transcription factor HvSNAC1 improves drought tolerance in barley (Hordeum vulgare L.). Mol. Breed. 33, 401–414.
-
- Alpert, P. (2006) Constraints of tolerance: why are desiccation‐tolerant organisms so small or rare? J. Exp. Biol. 209, 1575–1584. - PubMed
-
- Anjum, S.A. , Xie, X.Y. , Wang, L.C. , Saleem, M.F. , Man, C. and Lei, W. (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 6, 2026–2032.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

