Common and Potentially Prebiotic Origin for Precursors of Nucleotide Synthesis and Activation
- PMID: 28640999
- PMCID: PMC6326526
- DOI: 10.1021/jacs.7b01562
Common and Potentially Prebiotic Origin for Precursors of Nucleotide Synthesis and Activation
Abstract
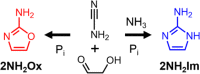
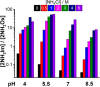
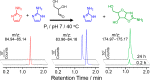
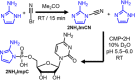
We have recently shown that 2-aminoimidazole is a superior nucleotide activating group for nonenzymatic RNA copying. Here we describe a prebiotic synthesis of 2-aminoimidazole that shares a common mechanistic pathway with that of 2-aminooxazole, a previously described key intermediate in prebiotic nucleotide synthesis. In the presence of glycolaldehyde, cyanamide, phosphate and ammonium ion, both 2-aminoimidazole and 2-aminooxazole are produced, with higher concentrations of ammonium ion and acidic pH favoring the former. Given a 1:1 mixture of 2-aminoimidazole and 2-aminooxazole, glyceraldehyde preferentially reacts and cyclizes with the latter, forming a mixture of pentose aminooxazolines, and leaving free 2-aminoimidazole available for nucleotide activation. The common synthetic origin of 2-aminoimidazole and 2-aminooxazole and their distinct reactivities are suggestive of a reaction network that could lead to both the synthesis of RNA monomers and to their subsequent chemical activation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Fahrenbach A. C. Pure Appl. Chem. 2015, 87, 205.10.1515/pac-2014-1004. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

