Rare Copy Number Variants in NRXN1 and CNTN6 Increase Risk for Tourette Syndrome
- PMID: 28641109
- PMCID: PMC5568251
- DOI: 10.1016/j.neuron.2017.06.010
Rare Copy Number Variants in NRXN1 and CNTN6 Increase Risk for Tourette Syndrome
Abstract
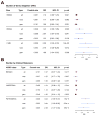
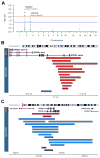
Tourette syndrome (TS) is a model neuropsychiatric disorder thought to arise from abnormal development and/or maintenance of cortico-striato-thalamo-cortical circuits. TS is highly heritable, but its underlying genetic causes are still elusive, and no genome-wide significant loci have been discovered to date. We analyzed a European ancestry sample of 2,434 TS cases and 4,093 ancestry-matched controls for rare (< 1% frequency) copy-number variants (CNVs) using SNP microarray data. We observed an enrichment of global CNV burden that was prominent for large (> 1 Mb), singleton events (OR = 2.28, 95% CI [1.39-3.79], p = 1.2 × 10-3) and known, pathogenic CNVs (OR = 3.03 [1.85-5.07], p = 1.5 × 10-5). We also identified two individual, genome-wide significant loci, each conferring a substantial increase in TS risk (NRXN1 deletions, OR = 20.3, 95% CI [2.6-156.2]; CNTN6 duplications, OR = 10.1, 95% CI [2.3-45.4]). Approximately 1% of TS cases carry one of these CNVs, indicating that rare structural variation contributes significantly to the genetic architecture of TS.
Keywords: CNTN6; NRXN1; Tourette Syndrome; copy number variation; genetics; neurodevelopmental disorders; structural variation; tic disorders.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Complex genetics of Tourette's Syndrome: Piecing the puzzle.Mov Disord. 2017 Dec;32(12):1685. doi: 10.1002/mds.27218. Epub 2017 Nov 13. Mov Disord. 2017. PMID: 29131391 No abstract available.
References
-
- Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, Mathews CA, Pauls DL, Rasin MR, Gunel M, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science. 2005;310:317–320. - PubMed
-
- Browne HA, Hansen SN, Buxbaum JD, Gair SL, Nissen JB, Nikolajsen KH, Schendel DE, Reichenberg A, Parner ET, Grice DE. Familial clustering of tic disorders and obsessive-compulsive disorder. JAMA Psychiatry. 2015;72:359–366. - PubMed
-
- Browne HA, Modabbernia A, Buxbaum JD, Hansen SN, Schendel DE, Parner ET, Reichenberg A, Grice DE. Prenatal Maternal Smoking and Increased Risk for Tourette Syndrome and Chronic Tic Disorders. J Am Acad Child Adolesc Psychiatry. 2016;55:784–791. - PubMed
-
- Burd L, Li Q, Kerbeshian J, Klug MG, Freeman RD. Tourette syndrome and comorbid pervasive developmental disorders. J Child Neurol. 2009;24:170–175. - PubMed
MeSH terms
Substances
Grants and funding
- R01 NS102371/NS/NINDS NIH HHS/United States
- K23 MH085057/MH/NIMH NIH HHS/United States
- U54 HD087101/HD/NICHD NIH HHS/United States
- R01 NS105746/NS/NINDS NIH HHS/United States
- U01 NS040024/NS/NINDS NIH HHS/United States
- R01 NS016648/NS/NINDS NIH HHS/United States
- K02 NS085048/NS/NINDS NIH HHS/United States
- R01 MH085548/MH/NIMH NIH HHS/United States
- R01 MH096767/MH/NIMH NIH HHS/United States
- P30 NS062691/NS/NINDS NIH HHS/United States
- R01 NS040024/NS/NINDS NIH HHS/United States
- U01 MH109514/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

