Sequential vs concurrent epirubicin and docetaxel as adjuvant chemotherapy for high-risk, node-negative, early breast cancer: an interim analysis of a randomised phase III study from the Hellenic Oncology Research Group
- PMID: 28641315
- PMCID: PMC5584474
- DOI: 10.1038/bjc.2017.158
Sequential vs concurrent epirubicin and docetaxel as adjuvant chemotherapy for high-risk, node-negative, early breast cancer: an interim analysis of a randomised phase III study from the Hellenic Oncology Research Group
Abstract
Background: Sequential anthracyclines and taxanes are standard adjuvant chemotherapy for patients with high-risk axillary node-positive breast cancer. We compared a sequential to a concurrent regimen in high-risk node-negative early breast cancer.
Methods: Patients were eligible if they had tumours >2 cm or T1c with two of the following characteristics: no oestrogen receptor (ER) and progesterone receptor (PR) expression, histological grade III, Ki67 >40% and vascular, lymphovascular or perineural invasion. They were randomised to receive four cycles of epirubicin 90 mg m-2 followed by four cycles of docetaxel 75 mg m-2 (sequential regimen) or six cycles of epirubicin 75 mg m-2 plus docetaxel 75 mg m-2 (concurrent regimen). All chemotherapy cycles were administered every 21 days with G-CSF prophylaxis only for the concurrent arm. The primary endpoint was disease-free survival (DFS).
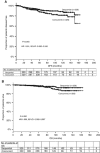
Results: Between 2001 and 2013, 658 women received the sequential (n=329) or the concurrent (n=329) regimen. The median age was 53 years, 43.9% of the patients were premenopausal and of the tumours 44.2% were ⩽2 cm, 52.7% histological grade 3 and 35.3% hormone receptor-negative. After a median follow-up of 70.5 months, there were 29 (8.8%) vs 42 (12.8%) disease relapses (P=0.102) and 11 (3.3%) vs 19 (5.8%) deaths (P=0.135), in the sequential and concurrent arm, respectively. The 5-year DFS rates were 92.6% vs 88.2% for sequential and concurrent arm, respectively (hazard ratio (HR): 1.591; 95% confidence interval (CI): 0.990-2.556; P=0.055). Toxicity included grade 2-4 neutropenia in 54% vs 41% (P=0.001), febrile neutropenia 2.7% vs 6.1% (P=0.06), nausea/vomiting 18.5% vs 12.4% (P=0.03) of patients in the sequential and concurrent arm. There were no toxic deaths.
Conclusions: Sequential compared with the concurrent administration of anthracyclines and taxanes is associated with a non-significant but possibly clinically meaningful improvement in DFS. In the era of molecular selection of patients for adjuvant chemotherapy, this study offers valuable information for the optimal administration of anthracyclines and taxanes in patients with node-negative disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Sequential versus concurrent chemotherapy for adjuvant breast cancer: does dose intensity matter?Br J Cancer. 2017 Jul 11;117(2):157-300. doi: 10.1038/bjc.2017.176. Epub 2017 Jun 22. Br J Cancer. 2017. PMID: 28641309 Free PMC article. No abstract available.
References
-
- Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE, Jacobs SA, Robert NJ, Atkins JN, O'Shaughnessy J, Dang CT, Gomez HL, Fehrenbacher L, Vukelja SJ, Lyss AP, Devchand Paul D, Brufsky AM, Swain SM, Mamounas EP, Jones SE, Wolmark N (2017) Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP-B49 (NRG Oncology). J Clin Oncol doi:10.1200/JCO.2016.71.4147. - PubMed
-
- Cardoso F, van’t Veer L, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, Glas AM, Golfinopoulos V, Goulioti T, Knox S, Matos E, Meulemans B, Neijenhuis PA, Nitz U, Passalacqua R, Ravdin P, Rubio IT, Saghatchian M, Smilde TJ, Sotiriou C, Stork L, Straehle C, Thomas G, Thompson AM, van der Hoeven JM, Vuylsteke P, Bernards R, Tryfonidis K, Rutgers E, Piccart M MINDACT Investigators (2016) 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375: 717–729. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K (2012) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet 379: 432–444. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials