Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases
- PMID: 28642117
- PMCID: PMC5677555
- DOI: 10.1016/j.pharmthera.2017.06.006
Soluble epoxide hydrolase as a therapeutic target for pain, inflammatory and neurodegenerative diseases
Abstract
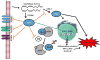
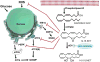
Eicosanoids are biologically active lipid signaling molecules derived from polyunsaturated fatty acids. Many of the actions of eicosanoid metabolites formed by cyclooxygenase and lipoxygenase enzymes have been characterized, however, the epoxy-fatty acids (EpFAs) formed by cytochrome P450 enzymes are newly described by comparison. The EpFA metabolites modulate a diverse set of physiologic functions that include inflammation and nociception among others. Regulation of EpFAs occurs primarily via release, biosynthesis and enzymatic transformation by the soluble epoxide hydrolase (sEH). Targeting sEH with small molecule inhibitors has enabled observation of the biological activity of the EpFAs in vivo in animal models, greatly contributing to the overall understanding of their role in the inflammatory response. Their role in modulating inflammation has been demonstrated in disease models including cardiovascular pathology and inflammatory pain, but extends to neuroinflammation and neuroinflammatory disease. Moreover, while EpFAs demonstrate activity against inflammatory pain, interestingly, this action extends to blocking chronic neuropathic pain as well. This review outlines the role of modulating sEH and the biological action of EpFAs in models of pain and inflammatory diseases.
Keywords: Alzheimer's disease; Depression; Epoxy-fatty acids (EpFAs); Inflammatory pain; Neuropathic pain; Soluble epoxide hydrolase (sEH).
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



References
-
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular Characterization of an Arachidonic Acid Epoxygenase in Rat Brain Astrocytes. Stroke. 1996;27:971–979. - PubMed
-
- Amruthesh SC, Falck JR, Ellis EF. Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem. 1992;58:503–510. - PubMed
-
- Bazan NG, Birkle DL, Tang W, Reddy TS. The accumulation of free arachidonic acid, diacylglycerols, prostaglandins, and lipoxygenase reaction products in the brain during experimental epilepsy. Adv Neurol. 1986;44:879–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

