Effects of Intensive BP Control in CKD
- PMID: 28642330
- PMCID: PMC5576945
- DOI: 10.1681/ASN.2017020148
Effects of Intensive BP Control in CKD
Abstract
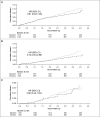
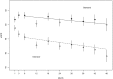
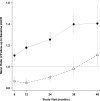
The appropriate target for BP in patients with CKD and hypertension remains uncertain. We report prespecified subgroup analyses of outcomes in participants with baseline CKD in the Systolic Blood Pressure Intervention Trial. We randomly assigned participants to a systolic BP target of <120 mm Hg (intensive group; n=1330) or <140 mm Hg (standard group; n=1316). After a median follow-up of 3.3 years, the primary composite cardiovascular outcome occurred in 112 intensive group and 131 standard group CKD participants (hazard ratio [HR], 0.81; 95% confidence interval [95% CI], 0.63 to 1.05). The intensive group also had a lower rate of all-cause death (HR, 0.72; 95% CI, 0.53 to 0.99). Treatment effects did not differ between participants with and without CKD (P values for interactions ≥0.30). The prespecified main kidney outcome, defined as the composite of ≥50% decrease in eGFR from baseline or ESRD, occurred in 15 intensive group and 16 standard group participants (HR, 0.90; 95% CI, 0.44 to 1.83). After the initial 6 months, the intensive group had a slightly higher rate of change in eGFR (-0.47 versus -0.32 ml/min per 1.73 m2 per year; P<0.03). The overall rate of serious adverse events did not differ between treatment groups, although some specific adverse events occurred more often in the intensive group. Thus, among patients with CKD and hypertension without diabetes, targeting an SBP<120 mm Hg compared with <140 mm Hg reduced rates of major cardiovascular events and all-cause death without evidence of effect modifications by CKD or deleterious effect on the main kidney outcome.
Keywords: blood pressure; cardiovascular disease; chronic kidney disease; glomerular filtration rate; hypertension; mortality.
Copyright © 2017 by the American Society of Nephrology.
Figures

Comment in
-
BP Targets in CKD, Mortality, and SPRINT: What Have We Learned?J Am Soc Nephrol. 2017 Sep;28(9):2561-2563. doi: 10.1681/ASN.2017060652. Epub 2017 Jul 20. J Am Soc Nephrol. 2017. PMID: 28729287 Free PMC article. No abstract available.
References
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 - PubMed
-
- Bakris GL, Ritz E: The message for world kidney day 2009: Hypertension and kidney disease: A marriage that should be prevented. Kidney Int 75: 449–452, 2009 - PubMed
-
- Anderson AH, Yang W, Townsend RR, Pan Q, Chertow GM, Kusek JW, Charleston J, He J, Kallem R, Lash JP, Miller ER 3rd, Rahman M, Steigerwalt S, Weir M, Wright JT Jr., Feldman HI; Chronic Renal Insufficiency Cohort Study Investigators : Time-updated systolic blood pressure and the progression of chronic kidney disease: A cohort study. Ann Intern Med 162: 258–265, 2015 - PMC - PubMed
-
- Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 - PubMed
-
- Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

