Pomalidomide, bortezomib and low-dose dexamethasone in lenalidomide-refractory and proteasome inhibitor-exposed myeloma
- PMID: 28642620
- PMCID: PMC5729338
- DOI: 10.1038/leu.2017.173
Pomalidomide, bortezomib and low-dose dexamethasone in lenalidomide-refractory and proteasome inhibitor-exposed myeloma
Erratum in
-
Correction: Pomalidomide, bortezomib, and low-dose dexamethasone in lenalidomide-refractory and proteasome inhibitor-exposed myeloma.Leukemia. 2018 Oct;32(10):2305. doi: 10.1038/s41375-018-0235-5. Leukemia. 2018. PMID: 30218008 Free PMC article.
Abstract
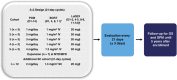
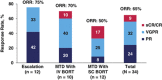
This phase 1 dose-escalation study evaluated pomalidomide, bortezomib (subcutaneous (SC) or intravenous (IV)) and low-dose dexamethasone (LoDEX) in lenalidomide-refractory and proteasome inhibitor-exposed relapsed or relapsed and refractory multiple myeloma (RRMM). In 21-day cycles, patients received pomalidomide (1-4 mg days 1-14), bortezomib (1-1.3 mg/m2 days 1, 4, 8 and 11 for cycles 1-8; days 1 and 8 for cycle ⩾9) and LoDEX. Primary endpoint was to determine the maximum tolerated dose (MTD). Thirty-four patients enrolled: 12 during escalation, 10 in the MTD IV bortezomib cohort and 12 in the MTD SC bortezomib cohort. Patients received a median of 2 prior lines of therapy; 97% bortezomib exposed. With no dose-limiting toxicities, MTD was defined as the maximum planned dose: pomalidomide 4 mg, bortezomib 1.3 mg/m2 and LoDEX. All patients discontinued treatment by data cutoff (2 April 2015). The most common grade 3/4 treatment-emergent adverse events were neutropenia (44%) and thrombocytopenia (26%), which occurred more frequently with IV than SC bortezomib. No grade 3/4 peripheral neuropathy or deep vein thrombosis was reported. Overall response rate was 65%. Median duration of response was 7.4 months. Pomalidomide, bortezomib and LoDEX was well tolerated and effective in lenalidomide-refractory and bortezomib-exposed patients with RRMM.
Trial registration: ClinicalTrials.gov NCT01734928.
Conflict of interest statement
PGR has served on advisory committees for Celgene, Novartis, Millennium and Takeda. CCH, YAE and JR have nothing to disclose. NSR has served as a consultant for Celgene, Takeda, Bristol-Myers Squibb, Amgen, Onyx, Millennium and Novartis and has received research funding from AstraZeneca, Eli Lilly and Acetylon. DSS has served on speakers bureaus for Celgene, Amgen, Takeda, Novartis and Merck. SL has served as a consultant for and received research funding from Celgene, Millennium, Novartis, Bristol-Myers Squibb, Onyx and Janssen. JL has received research funding from Celgene, Onyx, Millennium and Novartis. DHV has served on speakers bureaus for Celgene and has received research funding from Idera Pharmaceuticals. AKN has served as a consultant for Onyx and Spectrum Pharmaceuticals. DD has served on speakers bureaus for Celgene and Takeda. MHZ, YL and MSC are employees of and hold equity ownership in Celgene. AB, JH and LW are employees of Celgene. KCA has served as a consultant for Celgene, Millennium, Bristol-Myers Squibb and Gilead and has equity ownership in Acetylon and Oncocorp.
Figures
References
-
- Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc 2004; 79: 867–874. - PubMed
-
- Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai Y et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood 2000; 96: 2943–2950. - PubMed
-
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood 2002; 99: 4525–4530. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

