Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line
- PMID: 28642704
- PMCID: PMC5462925
- DOI: 10.3389/fphar.2017.00336
Quercetin, a Lead Compound against Type 2 Diabetes Ameliorates Glucose Uptake via AMPK Pathway in Skeletal Muscle Cell Line
Abstract
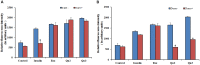
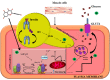
Herein we investigated the molecular mechanism of action of the citrus flavonoid, quercetin in skeletal muscle cells (L6 myotubes). Taking advantage of protein kinase inhibitors, we proved that the effect of quercetin on 2-NBDG uptake in L6 myotubes was not through insulin signaling pathway, but through adenosine monophosphate kinase (AMPK) pathway and its downstream target p38 MAPK. An increase in the cellular AMP to ATP ratio on pretreatment may account for AMPK activation which was coupled with a transient change in mitochondrial membrane potential. In addition, quercetin triggered a rise in intracellular calcium suggesting that calcium-calmodulin mediated protein kinase (CaMKK) may also be involved. Quercetin shared a similar mechanism with the well-known drug metformin, highlighting it as a promising compound for the management of type 2 diabetes. The AMPK signaling pathway could contribute to correction of insulin resistance through bypassing the insulin-regulated system for GLUT4 translocation.
Keywords: AMPK; CaMMK; L6 myotubes; quercetin; type 2 diabetes.
Figures

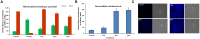
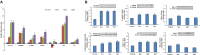
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

