Safety/tolerability of the anti-semaphorin 4D Antibody VX15/2503 in a randomized phase 1 trial
- PMID: 28642891
- PMCID: PMC5473956
- DOI: 10.1212/NXI.0000000000000367
Safety/tolerability of the anti-semaphorin 4D Antibody VX15/2503 in a randomized phase 1 trial
Abstract
Objective: To evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of VX15/2503 in a randomized, single-dose, dose-escalation, double-blind, placebo-controlled study enrolling adult patients with MS.
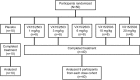
Methods: Single IV doses of VX15/2503 or placebo were administered. Ten patients each were randomized (4:1 randomization ratio) into 5 ascending dose cohorts of 1, 3, 6, 10, or 20 mg/kg. Safety, immunogenicity, PK/PD, MRI, ECG, and lymphocyte subset levels were evaluated. A Dose Escalation Safety Committee (DESC) approved each dose escalation.
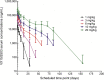
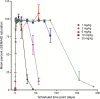
Results: VX15/2503 was well tolerated, and all participants completed the study. Antibody treatment-related adverse events were primarily grade 1 or 2 and included urinary tract infection (12.5%) and muscle weakness, contusion, and insomnia (each 7.5%). No dose-limiting toxicities were observed, and no maximum tolerated dose was determined. One subject (20 mg/kg) experienced disease relapse 3 months before study entry and exhibited a grade 3 (nonserious) increase in brain lesions by day 29, possibly related to VX15/2503. Twenty-nine patients exhibited human anti-humanized antibody responses; 5 with titer ≥100. No anti-VX15/2503 antibody responses were fully neutralizing. VX15/2503 Cmax, area under the time-concentration curve, and mean half-life increased with dose level; at 20 mg/kg, the T1/2 was 20 days. Cellular SEMA4D saturation occurred at serum antibody concentrations ≤0.3 μg/mL, resulting in decreased cSEMA4D expression. At 20 mg/kg, cSEMA4D saturation persisted for ≥155 days. Total sSEMA4D levels increased with dose level and declined with antibody clearance.
Conclusions: These results support the continued investigation of VX15/2503 in neurodegenerative diseases.
Clinicaltrialsgov identifier: NCT01764737.
Classification of evidence: This study provides Class III evidence that anti-semaphorin 4D antibody VX15/2503 at various doses was safe and well tolerated vs placebo, although an increase in treatment-emergent adverse events in the treatment group could not be excluded (risk difference -0.7%, 95% CI -28.0% to 32.7%).
Figures
References
-
- Southwell AL, Franciosi S, Villanueva EB, et al. Anti-semaphorin 4D immunotherapy ameliorates neuropathology and some cognitive impairment in the YAC128 mouse model of Huntington disease. Neuro Biol Dis 2015;76:46–56. - PubMed
-
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol 2008;9:17–23. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
