Dissociation of Down syndrome and Alzheimer's disease effects with imaging
- PMID: 28642933
- PMCID: PMC5477635
- DOI: 10.1016/j.trci.2016.02.004
Dissociation of Down syndrome and Alzheimer's disease effects with imaging
Abstract
Introduction: Down Syndrome (DS) adults experience accumulation of Alzheimer's disease (AD)-like amyloid plaques and tangles and a high incidence of dementia and could provide an enriched population to study AD-targeted treatments. However, to evaluate effects of therapeutic intervention, it is necessary to dissociate the contributions of DS and AD from overall phenotype. Imaging biomarkers offer the potential to characterize and stratify patients who will worsen clinically but have yielded mixed findings in DS subjects.
Methods: We evaluated 18F fluorodeoxyglucose positron emission tomography (PET), florbetapir PET, and structural magnetic resonance (sMR) image data from 12 nondemented DS adults using advanced multivariate machine learning methods.
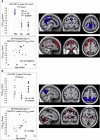
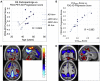
Results: Our results showed distinctive patterns of glucose metabolism and brain volume enabling dissociation of DS and AD effects. AD-like pattern expression corresponded to amyloid burden and clinical measures.
Discussion: These findings lay groundwork to enable AD clinical trials with characterization and disease-specific tracking of DS adults.
Keywords: AV-45; Alzheimer’s; Amyloid; Biomarker initiative; Classifier; Clinical trials; DSBI; Down syndrome; FDG; Glucose metabolism; Imaging; MRI; NPAIRS; PET; Prodromal.
Figures



References
-
- Ness S., Rafii M., Aisen P., Krams M., Silverman W., Manji H. Down's syndrome and Alzheimer's disease: towards secondary prevention. Nat Rev Drug Discov. 2012;11:655–656. - PubMed
-
- Drzezga A., Lautenschlager N., Siebner H., Riemenschneider M., Willoch F., Minoshima S. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–1113. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
