Cysteamine revisited: repair of arginine to cysteine mutations
- PMID: 28643139
- PMCID: PMC5740875
- DOI: 10.1007/s10545-017-0060-4
Cysteamine revisited: repair of arginine to cysteine mutations
Abstract
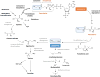
Cysteamine is a small aminothiol endogenously derived from coenzyme A degradation. For some decades, synthetic cysteamine has been employed for the treatment of cystinosis, and new uses of the drug continue to emerge. In this review, we discuss the role of cysteamine in cellular and extracellular homeostasis and focus on the potential use of aminothiols to reconstitute the function of proteins harboring arginine (Arg) to cysteine (Cys) mutations, via repair of the Cys residue into a moiety that introduces an amino group, as seen in basic amino acid residues Lys and Arg. Cysteamine has been utilized in vitro and ex vivo in four different genetic disorders, and thus provides "proof of principle" that aminothiols can modify Cys residues. Other aminothiols such as mercaptoethylguanidine (MEG) with closer structural resemblance to the guanidinium moiety of Arg are under examination for their predicted enhanced capacity to reconstitute loss of function. Although the use of aminothiols holds clinical potential, more studies are required to refine specificity and treatment design. The efficacy of aminothiols to target proteins may vary substantially depending on their specific extracellular and intracellular locations. Redox potential, pH, and specific aminothiol abundance in each physiological compartment are expected to influence the reactivity and turnover of cysteamine and analogous drugs. Upcoming research will require the use of suitable cell and animal models featuring Arg to Cys mutations. Since, in general, Arg to Cys changes comprise about 8% of missense mutations, repair of this specific mutation may provide promising avenues for many genetic diseases.
Keywords: Aminothiols; Cysteamine; Cysteine residue modification; Repair Arg to Cys mutations.
Conflict of interest statement
Figures
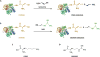

Similar articles
-
Small aminothiol compounds improve the function of Arg to Cys variant proteins: effect on the human cystathionine β-synthase p.R336C.Hum Mol Genet. 2015 Dec 20;24(25):7339-48. doi: 10.1093/hmg/ddv431. Epub 2015 Oct 12. Hum Mol Genet. 2015. PMID: 26464485
-
Effect of Cysteamine on Mutant ASL Proteins with Cysteine for Arginine Substitutions.Mol Diagn Ther. 2016 Apr;20(2):125-33. doi: 10.1007/s40291-015-0182-z. Mol Diagn Ther. 2016. PMID: 26745957
-
Formation and Properties of DNA Adducts Generated by Reactions of Abasic Sites with 1,2-Aminothiols Including Cysteamine, Cysteine Methyl Ester, and Peptides Containing N-Terminal Cysteine Residues.Chem Res Toxicol. 2024 Feb 19;37(2):395-406. doi: 10.1021/acs.chemrestox.3c00344. Epub 2024 Jan 5. Chem Res Toxicol. 2024. PMID: 38181204
-
Cystine depletion of cystinotic cells by aminothiols.Proc R Soc Med. 1977;70 Suppl 3(Suppl 3):37-40. doi: 10.1177/00359157770700S314. Proc R Soc Med. 1977. PMID: 122669 Free PMC article. Review. No abstract available.
-
Cysteine and related aminothiols in cardiovascular disease, obesity and insulin resistance.Adv Clin Chem. 2022;109:75-127. doi: 10.1016/bs.acc.2022.03.003. Epub 2022 Apr 29. Adv Clin Chem. 2022. PMID: 35953129 Review.
Cited by
-
RGS3 and IL1RAPL1 missense variants implicate defective neurotransmission in early-onset inherited schizophrenias.J Psychiatry Neurosci. 2022 Nov 1;47(6):E379-E390. doi: 10.1503/jpn.220070. Print 2022 Nov-Dec. J Psychiatry Neurosci. 2022. PMID: 36318984 Free PMC article.
-
Immunomodulatory effects of cysteamine and its potential use as a host-directed therapy for tuberculosis.Front Immunol. 2024 Oct 28;15:1411827. doi: 10.3389/fimmu.2024.1411827. eCollection 2024. Front Immunol. 2024. PMID: 39530101 Free PMC article.
-
The Spectrum of Mutations of Homocystinuria in the MENA Region.Genes (Basel). 2020 Mar 20;11(3):330. doi: 10.3390/genes11030330. Genes (Basel). 2020. PMID: 32245022 Free PMC article. Review.
-
A New and Rapid LC-MS/MS Method for the Determination of Cysteamine Plasma Levels in Cystinosis Patients.Pharmaceuticals (Basel). 2024 May 16;17(5):649. doi: 10.3390/ph17050649. Pharmaceuticals (Basel). 2024. PMID: 38794219 Free PMC article.
-
Chemistry and Biochemistry of Sulfur Natural Compounds: Key Intermediates of Metabolism and Redox Biology.Oxid Med Cell Longev. 2020 Sep 29;2020:8294158. doi: 10.1155/2020/8294158. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33062147 Free PMC article. Review.
References
-
- Bacq ZM, Dechamps G, Fischer P, et al. Protection against x-rays and therapy of radiation sickness with beta-mercaptoethylamine. Science. 1953;117:633–6. - PubMed
-
- Balmer C, Pandey AV, Rüfenacht V, et al. Mutations and polymorphisms in the human argininosuccinate lyase (ASL) gene. Hum Mutat. 2014;35:27–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

