Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis
- PMID: 28643167
- PMCID: PMC11107790
- DOI: 10.1007/s00018-017-2550-9
Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis
Abstract
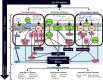
The gut microbiota is essential to health and has recently become a target for live bacterial cell biotherapies for various chronic diseases including metabolic syndrome, diabetes, obesity and neurodegenerative disease. Probiotic biotherapies are known to create a healthy gut environment by balancing bacterial populations and promoting their favorable metabolic action. The microbiota and its respective metabolites communicate to the host through a series of biochemical and functional links thereby affecting host homeostasis and health. In particular, the gastrointestinal tract communicates with the central nervous system through the gut-brain axis to support neuronal development and maintenance while gut dysbiosis manifests in neurological disease. There are three basic mechanisms that mediate the communication between the gut and the brain: direct neuronal communication, endocrine signaling mediators and the immune system. Together, these systems create a highly integrated molecular communication network that link systemic imbalances with the development of neurodegeneration including insulin regulation, fat metabolism, oxidative markers and immune signaling. Age is a common factor in the development of neurodegenerative disease and probiotics prevent many harmful effects of aging such as decreased neurotransmitter levels, chronic inflammation, oxidative stress and apoptosis-all factors that are proven aggravators of neurodegenerative disease. Indeed patients with Parkinson's and Alzheimer's diseases have a high rate of gastrointestinal comorbidities and it has be proposed by some the management of the gut microbiota may prevent or alleviate the symptoms of these chronic diseases.
Keywords: Gut microbiota; Gut-brain-axis; Neurodegeneration; Oxidative stress; Probiotics; Short-chain fatty acids.
Conflict of interest statement
The authors declare that there is no conflict of interest or competing financial interests.
Figures

References
-
- Forsythe P, Bienenstock J, Kunze WA. Microbial endocrinology: the microbiota–gut–brain axis in health and disease. New York: Springer; 2014. Vagal pathways for microbiome–brain–gut axis communication; pp. 115–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

