Treatment With Antipsychotics in Pregnancy: Changes in Drug Disposition
- PMID: 28643331
- PMCID: PMC5836849
- DOI: 10.1002/cpt.770
Treatment With Antipsychotics in Pregnancy: Changes in Drug Disposition
Abstract
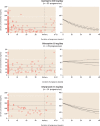
Although pregnancy is known to cause changes in drug pharmacokinetics, little is known about its impact on serum levels of antipsychotics. In this study we retrospectively assessed 201 routine serum antipsychotic therapeutic drug monitoring concentration measurements obtained from a total of 110 pregnancies in 103 women, and 512 measurements from the same women before and after pregnancy. Serum concentrations in the third trimester were significantly lower than baseline for quetiapine (-76%; confidence interval (CI), -83%, -66%; P < 0.001) and aripiprazole (-52%; CI, -62%, -39%; P < 0.001), but not for olanzapine (-9%; CI, -28%, +14%; P = 0.40). For the remaining antipsychotics (perphenazine, haloperidol, ziprasidone, risperidone, and clozapine), our dataset was limited, but it indicates that concentrations may decline at least for perphenazine and possibly also for haloperidol. Even though the clinical consequence of the serum concentrations decline remains to be elucidated, our results warrant close clinical monitoring throughout pregnancy, preferentially supported by therapeutic drug monitoring.
© 2017 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

References
-
- Chisolm, M.S. & Payne, J.L. Management of psychotropic drugs during pregnancy. BMJ 532, h5918 (2016). - PubMed
-
- Cohen, L.S. et al Reproductive safety of second‐generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry 173, 263–270 (2016). - PubMed
-
- Anderson, G.D. Pregnancy‐induced changes in pharmacokinetics: a mechanistic‐based approach. Clin. Pharmacokinet. 44, 989–1008 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

