Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus
- PMID: 28643775
- PMCID: PMC5490051
- DOI: 10.1038/ncomms15674
Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus
Abstract
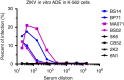
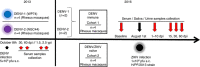
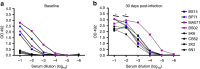
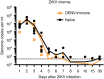
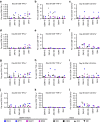
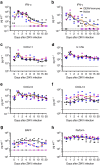
Zika virus (ZIKV) is a re-emerging virus that has recently spread into dengue virus (DENV) endemic regions and cross-reactive antibodies (Abs) could potentially affect ZIKV pathogenesis. Using DENV-immune serum, it has been shown in vitro that antibody-dependent enhancement (ADE) of ZIKV infection can occur. Here we study the effects of pre-existing DENV immunity on ZIKV infection in vivo. We infect two cohorts of rhesus macaques with ZIKV; one cohort has been exposed to DENV 2.8 years earlier and a second control cohort is naïve to flaviviral infection. Our results, while confirming ADE in vitro, suggest that pre-existing DENV immunity does not result in more severe ZIKV disease. Rather our results show a reduction in the number of days of ZIKV viremia compared to naïve macaques and that the previous exposure to DENV may result in modulation of the immune response without resulting in enhancement of ZIKV pathogenesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- WHO. WHO statement on the first meeting of the International Health Regulations (IHR, 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. http://www.who.int/mediacentre/news/statements/2016/1st-emergency-commit... (2016).
-
- Barba-Spaeth G. et al.. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 536, 48–53 (2016). - PubMed
-
- Green S. & Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 19, 429–436 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

