More Dose-dependent Side Effects with Mercaptopurine over Azathioprine in IBD Treatment Due to Relatively Higher Dosing
- PMID: 28644183
- PMCID: PMC5598908
- DOI: 10.1097/MIB.0000000000001163
More Dose-dependent Side Effects with Mercaptopurine over Azathioprine in IBD Treatment Due to Relatively Higher Dosing
Abstract
Background: There are substantial global differences in the preference for mercaptopurine (MP) or its prodrug azathioprine (AZA) as first-choice thiopurine to treat inflammatory bowel diseases. Studies comparing both agents are scarce. Our aim was to compare AZA and MP in thiopurine-naive patients with inflammatory bowel disease for the frequency of side effects and efficacy.
Methods: Post hoc analysis of the "Thiopurine response Optimization by Pharmacogenetic testing in Inflammatory bowel disease Clinics" (TOPIC) trial, in which thiopurine-naive patients with inflammatory bowel disease with an indication for a thiopurine were randomized for a genotype-based dose versus standard of care. For this study, Cox proportional hazard ratios (HRs) were calculated to compare AZA and MP for discontinuation rates within 5 months, incidence of hepatotoxicity, leukopenia, and gastrointestinal side effects. Treatment efficacy was compared by logistic regression.
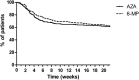
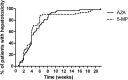
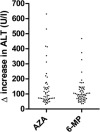
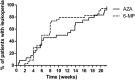
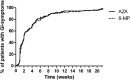
Results: Patient characteristics were similar for patients treated with AZA (n = 494, 64.4%) and MP (n = 273, 35.6%), yet patients with MP were relatively higher dosed compared with those on AZA. Discontinuation rates within 5 months were not different, 39.3% (AZA) and 38.1% (MP), HR 0.92 (95% confidence interval, 0.72-1.17; P = 0.50); however, patients on MP were more often subjected to dose reductions (30% versus 14%, P < 0.01). Higher rates of hepatotoxicity, HR 1.93 (95% confidence interval, 1.35-2.76; P < 0.01) and leukopenia, HR 2.55 (95% confidence interval, 1.51-4.30; P < 0.01) were observed with MP, which annulled in a secondary analysis with adjustment for the higher dose and metabolite levels.
Conclusions: Patients treated with MP were relatively higher dosed, which resulted in more dose-dependent side effects and a higher rate of dose reductions.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
References
-
- van Gelder T, van Schaik RH, Hesselink DA. Pharmacogenetics and immunosuppressive drugs in solid organ transplantation. Nat Rev Nephrol. 2014;10:725–731. - PubMed
-
- Lamers MM, van Oijen MG, Pronk M, et al. Treatment options for autoimmune hepatitis: a systematic review of randomized controlled trials. J Hepatol. 2010;53:191–198. - PubMed
-
- Kirchgesner J, Lemaitre M, Rudnichi A, et al. Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: analysis of the French administrative health databases 2009–2014. Aliment Pharmacol Ther. 2017;45:37–49. - PubMed
-
- Rispo A, Testa A, De Palma GD, et al. Different profile of efficacy of thiopurines in ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2015;21:2570–2575. - PubMed
-
- Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn's disease. Gastroenterology. 2013;145:1459–1463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

