Metal Ion-Loaded Nanofibre Matrices for Calcification Inhibition in Polyurethane Implants
- PMID: 28644382
- PMCID: PMC5618273
- DOI: 10.3390/jfb8030022
Metal Ion-Loaded Nanofibre Matrices for Calcification Inhibition in Polyurethane Implants
Abstract
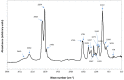
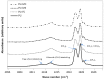
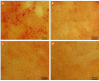
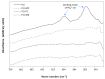
Pathologic calcification leads to structural deterioration of implant materials via stiffening, stress cracking, and other structural disintegration mechanisms, and the effect can be critical for implants intended for long-term or permanent implantation. This study demonstrates the potential of using specific metal ions (MI)s for inhibiting pathological calcification in polyurethane (PU) implants. The hypothesis of using MIs as anti-calcification agents was based on the natural calcium-antagonist role of Mg2+ ions in human body, and the anti-calcification effect of Fe3+ ions in bio-prosthetic heart valves has previously been confirmed. In vitro calcification results indicated that a protective covering mesh of MI-doped PU can prevent calcification by preventing hydroxyapatite crystal growth. However, microstructure and mechanical characterisation revealed oxidative degradation effects from Fe3+ ions on the mechanical properties of the PU matrix. Therefore, from both a mechanical and anti-calcification effects point of view, Mg2+ ions are more promising candidates than Fe3+ ions. The in vitro MI release experiments demonstrated that PU microphase separation and the structural design of PU-MI matrices were important determinants of release kinetics. Increased phase separation in doped PU assisted in consistent long-term release of dissolved MIs from both hard and soft segments of the PU. The use of a composite-sandwich mesh design prevented an initial burst release which improved the late (>20 days) release rate of MIs from the matrix.
Keywords: Alizarin red S staining; Von Kossa method; anti-calcification; calcification; hydroxyapatite; magnesium; metal ion; nanofibre matrix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Electrospun polyurethane/hydroxyapatite bioactive scaffolds for bone tissue engineering: the role of solvent and hydroxyapatite particles.J Mech Behav Biomed Mater. 2014 Nov;39:95-110. doi: 10.1016/j.jmbbm.2014.06.019. Epub 2014 Jul 18. J Mech Behav Biomed Mater. 2014. PMID: 25117379
-
Prevention of calcification of glutaraldehyde pretreated bovine pericardium through controlled release polymeric implants: studies of Fe3+, Al3+, protamine sulphate and levamisole.Biomaterials. 1990 Nov;11(9):718-23. doi: 10.1016/0142-9612(90)90034-n. Biomaterials. 1990. PMID: 2128616
-
Composite elastomeric polyurethane scaffolds incorporating small intestinal submucosa for soft tissue engineering.Acta Biomater. 2017 Sep 1;59:45-57. doi: 10.1016/j.actbio.2017.05.041. Epub 2017 May 17. Acta Biomater. 2017. PMID: 28528117
-
Biomedical applications of polyurethanes: implications of failure mechanisms.J Biomater Appl. 1988 Oct;3(2):207-27. doi: 10.1177/088532828800300204. J Biomater Appl. 1988. PMID: 3060586 Review.
-
Founder's Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, 1999. Tissue heart valves: current challenges and future research perspectives.J Biomed Mater Res. 1999 Dec 15;47(4):439-65. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. J Biomed Mater Res. 1999. PMID: 10497280 Review.
Cited by
-
Prosthetic vascular grafts engineered to combat calcification: Progress and future directions.Biotechnol Bioeng. 2023 Apr;120(4):953-969. doi: 10.1002/bit.28316. Epub 2022 Dec 28. Biotechnol Bioeng. 2023. PMID: 36544433 Free PMC article. Review.
-
A critical review of fibrous polyurethane-based vascular tissue engineering scaffolds.J Biol Eng. 2022 Mar 24;16(1):6. doi: 10.1186/s13036-022-00286-9. J Biol Eng. 2022. PMID: 35331305 Free PMC article. Review.
References
-
- Thoma R.J., Phillips R.E. The role of material surface chemistry in implant device calcification: A hypothesis. J. Heart Valve Dis. 1995;4:214–221. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

