CH/π Interactions in Carbohydrate Recognition
- PMID: 28644385
- PMCID: PMC6152320
- DOI: 10.3390/molecules22071038
CH/π Interactions in Carbohydrate Recognition
Abstract
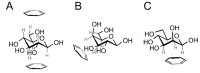
Many carbohydrate-binding proteins contain aromatic amino acid residues in their binding sites. These residues interact with carbohydrates in a stacking geometry via CH/π interactions. These interactions can be found in carbohydrate-binding proteins, including lectins, enzymes and carbohydrate transporters. Besides this, many non-protein aromatic molecules (natural as well as artificial) can bind saccharides using these interactions. Recent computational and experimental studies have shown that carbohydrate-aromatic CH/π interactions are dispersion interactions, tuned by electrostatics and partially stabilized by a hydrophobic effect in solvated systems.
Keywords: CH/π interactions; carbohydrate-protein interactions; interaction energy; lectins; non-canonical hydrogen bond.
Conflict of interest statement
The author declares no conflict of interest.
Figures


Similar articles
-
Stacking interactions between carbohydrate and protein quantified by combination of theoretical and experimental methods.PLoS One. 2012;7(10):e46032. doi: 10.1371/journal.pone.0046032. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056230 Free PMC article.
-
Substituent effects in synthetic lectins--exploring the role of CH-π interactions in carbohydrate recognition.J Org Chem. 2011 Aug 19;76(16):6548-57. doi: 10.1021/jo200755z. Epub 2011 Jul 18. J Org Chem. 2011. PMID: 21732662
-
The importance of CH/pi interactions to the function of carbohydrate binding proteins.Protein Pept Lett. 2002 Jun;9(3):195-209. doi: 10.2174/0929866023408751. Protein Pept Lett. 2002. PMID: 12144516 Review.
-
Carbohydrate-aromatic interactions.Acc Chem Res. 2013 Apr 16;46(4):946-54. doi: 10.1021/ar300024d. Epub 2012 Jun 15. Acc Chem Res. 2013. PMID: 22704792
-
Lectin-carbohydrate interactions: different folds, common recognition principles.Trends Biochem Sci. 1997 Dec;22(12):462-7. doi: 10.1016/s0968-0004(97)01146-8. Trends Biochem Sci. 1997. PMID: 9433125 Review.
Cited by
-
The Role of Tryptophan in π Interactions in Proteins: An Experimental Approach.J Am Chem Soc. 2022 Aug 3;144(30):13815-13822. doi: 10.1021/jacs.2c04986. Epub 2022 Jul 22. J Am Chem Soc. 2022. PMID: 35868012 Free PMC article.
-
An overview on the synthesis of carbohydrate-based molecules with biological activity related to neurodegenerative diseases.RSC Med Chem. 2021 Sep 8;12(12):2001-2015. doi: 10.1039/d1md00217a. eCollection 2021 Dec 15. RSC Med Chem. 2021. PMID: 35028560 Free PMC article. Review.
-
The energetic landscape of CH-π interactions in protein-carbohydrate binding.Chem Sci. 2024 Dec 3;16(4):1746-1761. doi: 10.1039/d4sc06246a. eCollection 2025 Jan 22. Chem Sci. 2024. PMID: 39669175 Free PMC article.
-
Rational Design of TDP-43 Derived α-Helical Peptide Inhibitors: An In Silico Strategy to Prevent TDP-43 Aggregation in Neurodegenerative Disorders.ACS Chem Neurosci. 2024 Mar 20;15(6):1096-1109. doi: 10.1021/acschemneuro.3c00659. Epub 2024 Mar 11. ACS Chem Neurosci. 2024. PMID: 38466778 Free PMC article.
-
Protein-Ligand CH-π Interactions: Structural Informatics, Energy Function Development, and Docking Implementation.J Chem Theory Comput. 2023 Aug 22;19(16):5503-5515. doi: 10.1021/acs.jctc.3c00300. Epub 2023 Jul 26. J Chem Theory Comput. 2023. PMID: 37493980 Free PMC article.
References
-
- Karasová-Lipovová P., Strnad H., Spiwok V., Malá Š., Kralová B., Russell N.J. The Cloning, Purification and Characterisation of a Cold-Active β-Galactosidase from the Psychrotolerant Antarctic Bacterium Arthrobacter sp. C2-2. Enzym. Microb. Technol. 2003;33:836–844. doi: 10.1016/S0141-0229(03)00211-4. - DOI
-
- Petroková H., Vondráčková E., Skálová T., Dohnálek J., Lipovová P., Spiwok V., Strnad H., Králová B., Hašek J. Crystallization and Preliminary X-Ray Diffraction Analysis of Cold-Active β-Galactosidase from Arthrobacter sp. C2-2. Collect. Czech. Chem. Commun. 2005;70:124–132. doi: 10.1135/cccc20050124. - DOI
-
- Skálová T., Dohnálek J., Spiwok V., Lipovová P., Vondráčková E., Petroková H., Dušková J., Strnad H., Králová B., Hašek J. Cold-active β-Galactosidase from Arthrobacter sp. C2-2 Forms Compact 660 kDa Hexamers: Crystal Structure at 1.9 Å Resolution. J. Mol. Biol. 2005;353:282–294. doi: 10.1016/j.jmb.2005.08.028. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

