Enantioselective, intermolecular benzylic C-H amination catalysed by an engineered iron-haem enzyme
- PMID: 28644476
- PMCID: PMC5555633
- DOI: 10.1038/nchem.2783
Enantioselective, intermolecular benzylic C-H amination catalysed by an engineered iron-haem enzyme
Abstract
C-H bonds are ubiquitous structural units of organic molecules. Although these bonds are generally considered to be chemically inert, the recent emergence of methods for C-H functionalization promises to transform the way synthetic chemistry is performed. The intermolecular amination of C-H bonds represents a particularly desirable and challenging transformation for which no efficient, highly selective, and renewable catalysts exist. Here we report the directed evolution of an iron-containing enzymatic catalyst-based on a cytochrome P450 monooxygenase-for the highly enantioselective intermolecular amination of benzylic C-H bonds. The biocatalyst is capable of up to 1,300 turnovers, exhibits excellent enantioselectivities, and provides access to valuable benzylic amines. Iron complexes are generally poor catalysts for C-H amination: in this catalyst, the enzyme's protein framework confers activity on an otherwise unreactive iron-haem cofactor.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

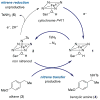
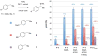

Comment in
-
Biocatalysis: Achieving aminase activity.Nat Chem Biol. 2017 Jul 18;13(8):817. doi: 10.1038/nchembio.2447. Nat Chem Biol. 2017. PMID: 28853731 No abstract available.
References
-
- Godula K, Sames D. C–H Bond Functionalization in Complex Organic Synthesis. Science. 2006;312:67–72. - PubMed
-
- Yamaguchi J, Yamaguchi AD, Itami K. C–H Bond Functionalization: Emerging Synthetic Tools for Natural Products and Pharmaceuticals. Angew Chem Int Ed. 2012;51:8960–9009. - PubMed
-
- Bertini I, Gray HB, Lippard SJ, Valentine JS, editors. Bioinorganic Chemistry. University Science Books; Mill Valley, CA: 1994.
-
- Zalatan DN, Du Bois J. Metal-Catalyzed Oxidations of C–H to C–N Bonds. Top Curr Chem. 2010;292:347–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

