Quantitative Insights into the Fast Pyrolysis of Extracted Cellulose, Hemicelluloses, and Lignin
- PMID: 28644517
- PMCID: PMC5582602
- DOI: 10.1002/cssc.201700984
Quantitative Insights into the Fast Pyrolysis of Extracted Cellulose, Hemicelluloses, and Lignin
Abstract
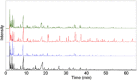
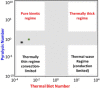
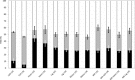
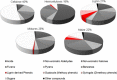
The transformation of lignocellulosic biomass into bio-based commodity chemicals is technically possible. Among thermochemical processes, fast pyrolysis, a relatively mature technology that has now reached a commercial level, produces a high yield of an organic-rich liquid stream. Despite recent efforts to elucidate the degradation paths of biomass during pyrolysis, the selectivity and recovery rates of bio-compounds remain low. In an attempt to clarify the general degradation scheme of biomass fast pyrolysis and provide a quantitative insight, the use of fast pyrolysis microreactors is combined with spectroscopic techniques (i.e., mass spectrometry and NMR spectroscopy) and mixtures of unlabeled and 13 C-enriched materials. The first stage of the work aimed to select the type of reactor to use to ensure control of the pyrolysis regime. A comparison of the chemical fragmentation patterns of "primary" fast pyrolysis volatiles detected by using GC-MS between two small-scale microreactors showed the inevitable occurrence of secondary reactions. In the second stage, liquid fractions that are also made of primary fast pyrolysis condensates were analyzed by using quantitative liquid-state 13 C NMR spectroscopy to provide a quantitative distribution of functional groups. The compilation of these results into a map that displays the distribution of functional groups according to the individual and main constituents of biomass (i.e., hemicelluloses, cellulose and lignin) confirmed the origin of individual chemicals within the fast pyrolysis liquids.
Keywords: NMR spectroscopy; biomass; isotopic labeling; polymers; reaction mechanisms.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures





References
-
- Meier D., Van De Beld B., Bridgwater A. V., Elliott D. C., Oasmaa A., Preto F., Renewable Sustainable Energy Rev. 2013, 20, 619–641.
-
- Maity S. K., Renewable Sustainable Energy Rev. 2015, 43, 1446–1466.
-
- Bridgwater A. V., Chem. Eng. J. 2003, 91, 87–102.
-
- Liu C., Wang H., Karim A. M., Sun J., Wang Y., Chem. Soc. Rev. 2014, 43, 7594–7623. - PubMed
-
- Zhou C.-H., Xia X., Lin C.-X., Tong D.-S., Beltramini J., Chem. Soc. Rev. 2011, 40, 5588–5617. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

