An unstructured loop that is critical for interactions of the stalk domain of Drp1 with saturated phosphatidic acid
- PMID: 28644713
- PMCID: PMC6204998
- DOI: 10.1080/21541248.2017.1321614
An unstructured loop that is critical for interactions of the stalk domain of Drp1 with saturated phosphatidic acid
Abstract
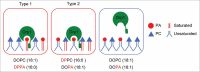
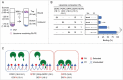
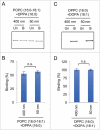
Dynamin-related protein 1 (Drp1) is a dynamin superfamily GTPase, which drives membrane constriction during mitochondrial division. To mediate mitochondrial division, Drp1 is recruited to the mitochondrial outer membrane and is assembled into the division machinery. We previously showed that Drp1 interacts with phosphatidic acid (PA) and saturated phospholipids in the mitochondrial membrane, and this interaction restrains Drp1 in initiating the constriction of mitochondria. Here, we show that the role of saturated acyl chains of phospholipids is independent of their contribution to the membrane curvature or lipid packing suggesting their direct interaction with Drp1. We further show that an unstructured loop in the stalk domain of Drp1 is critical for interaction with unsaturated PA. Our data significantly advance our understanding of this unique protein-lipid interaction involved in mitochondrial division.
Keywords: GTPase; dynamin-related protein 1; lipid binding; liposomes; mitochondria; organelle dynamics; phosphatidic acid.
Figures


References
-
- Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 2012; 337:1062-5; PMID:22936770; https://doi.org/10.1126/science.1219855 - DOI - PMC - PubMed
-
- Pernas L, Scorrano L. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu Rev Physiol 2016; 78:505-31; PMID:26667075; https://doi.org/10.1146/annurev-physiol-021115-105011 - DOI - PubMed
-
- Friedman JR, Nunnari J. Mitochondrial form and function. Nature 2014; 505:335-43; PMID:24429632; https://doi.org/10.1038/nature12985 - DOI - PMC - PubMed
-
- Shutt TE, McBride HM. Staying cool in difficult times: Mitochondrial dynamics, quality control and the stress response. Biochim Et Biophys Acta 2012; PMID:22683990; https://doi.org/10.1016/j.bbamcr.2012.05.024 - DOI - PubMed
-
- Roy M, Reddy PH, Iijima M, Sesaki H. Mitochondrial division and fusion in metabolism. Curr Opin Cell Biol 2015; 33C:111-8; https://doi.org/10.1016/j.ceb.2015.02.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
