Real-world first-line treatment and overall survival in non-small cell lung cancer without known EGFR mutations or ALK rearrangements in US community oncology setting
- PMID: 28644837
- PMCID: PMC5482433
- DOI: 10.1371/journal.pone.0178420
Real-world first-line treatment and overall survival in non-small cell lung cancer without known EGFR mutations or ALK rearrangements in US community oncology setting
Abstract
Purpose: To establish a baseline for care and overall survival (OS) based upon contemporary first-line treatments prescribed in the era before the introduction of immune checkpoint inhibitors, for people with metastatic non-small cell lung cancer (NSCLC) without common actionable mutations.
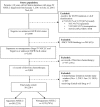
Methods: Using a nationally representative electronic health record data from the Flatiron dataset which included 162 practices from different regions in US, we identified patients (≥18 years old) newly diagnosed with stage IV NSCLC initiating first-line anticancer therapy (November 2012- January 2015, with follow-up through July 2015). Patients with documented epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) translocation were excluded. Anti-cancer drug therapy and overall survival were described overall, and by histology.
Results: A total of 2,014 patients with stage IV NSCLC without known EGFR or ALK genomic tumor aberrations initiated systemic anticancer therapy, 22% with squamous and 78% with nonsquamous histology. Their mean (SD) age was 67 (10) years, 55% were male, and 87% had a smoking history. In nonsquamous NSCLC, carboplatin plus pemetrexed either without (25.7%) or with bevacizumab (16%) were the most common regimens; 26.6% of nonsquamous patients receiving induction therapy also received continuation maintenance therapy. In squamous NSCLC, carboplatin plus paclitaxel (37.6%) or nab-paclitaxel (21.1%) were the most commonly used regimens. Overall median OS was 9.7 months (95% CI: 9.1, 10.3), 8.5 months (95% CI: 7.4, 10.0) for squamous, and 10.0 months (95% CI: 9.4, 10.8) for nonsquamous NSCLC.
Conclusion: The results provide context for evaluating the effect of shifting treatment patterns of NSCLC treatments on patient outcomes, and for community oncology benchmarking initiatives.
Conflict of interest statement
Figures

References
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. (eds). Surveillance Epidemiology and End Results (SEER) Program: SEER Cancer Statistics Review (CSR) 1975–2012 (updated August 20, 2015). Bethesda, MD: National Cancer Institute, 2015. http://seer.cancer.gov/csr/1975_2012/
-
- Herbst RS, Heymach JV, Lippman SM: Lung cancer. N Engl J Med 359:1367–1380, 2008. doi: 10.1056/NEJMra0802714 - DOI - PMC - PubMed
-
- SEER Cancer Statistics Factsheets: Lung and Bronchus Cancer, 2015. http://seer.cancer.gov/statfacts/html/lungb.html
-
- NCCN Guidelines: Non-small cell lung cancer version 7.2015, 2015. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
-
- Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S Jr, Brahmer JR, et al.: Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 33:3488–3515, 2015. doi: 10.1200/JCO.2015.62.1342 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

