AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome
- PMID: 28645211
- PMCID: PMC5587066
- DOI: 10.1210/jc.2017-00947
AKR1C3-Mediated Adipose Androgen Generation Drives Lipotoxicity in Women With Polycystic Ovary Syndrome
Abstract
Context: Polycystic ovary syndrome (PCOS) is a prevalent metabolic disorder occurring in up to 10% of women of reproductive age. PCOS is associated with insulin resistance and cardiovascular risk. Androgen excess is a defining feature of PCOS and has been suggested as causally associated with insulin resistance; however, mechanistic evidence linking both is lacking. We hypothesized that adipose tissue is an important site linking androgen activation and metabolic dysfunction in PCOS.
Methods: We performed a human deep metabolic in vivo phenotyping study examining the systemic and intra-adipose effects of acute and chronic androgen exposure in 10 PCOS women, in comparison with 10 body mass index-matched healthy controls, complemented by in vitro experiments.
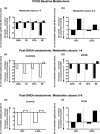
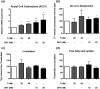
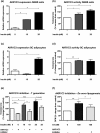
Results: PCOS women had increased intra-adipose concentrations of testosterone (P = 0.0006) and dihydrotestosterone (P = 0.01), with increased expression of the androgen-activating enzyme aldo-ketoreductase type 1 C3 (AKR1C3) (P = 0.04) in subcutaneous adipose tissue. Adipose glycerol levels in subcutaneous adipose tissue microdialysate supported in vivo suppression of lipolysis after acute androgen exposure in PCOS (P = 0.04). Mirroring this, nontargeted serum metabolomics revealed prolipogenic effects of androgens in PCOS women only. In vitro studies showed that insulin increased adipose AKR1C3 expression and activity, whereas androgen exposure increased adipocyte de novo lipid synthesis. Pharmacologic AKR1C3 inhibition in vitro decreased de novo lipogenesis.
Conclusions: These findings define an intra-adipose mechanism of androgen activation that contributes to adipose remodeling and a systemic lipotoxic metabolome, with intra-adipose androgens driving lipid accumulation and insulin resistance in PCOS. AKR1C3 represents a promising therapeutic target in PCOS.
Copyright © 2017 Endocrine Society
Figures



References
-
- Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333(13):853–861. - PubMed
-
- Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod. 2012;27(10):3067–3073. - PubMed
-
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18(6):774–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

