Identification of the flotillin-1/2 heterocomplex as a target of autoantibodies in bona fide multiple sclerosis
- PMID: 28645295
- PMCID: PMC5481867
- DOI: 10.1186/s12974-017-0900-z
Identification of the flotillin-1/2 heterocomplex as a target of autoantibodies in bona fide multiple sclerosis
Abstract
Background: Autoantibodies, in particular those against aquaporin-4 and myelin-oligodendrocyte glycoprotein (MOG), aid as biomarkers in the differential diagnosis of demyelination. Here, we report on discovery of autoantibodies against flotillin in patients with multiple sclerosis (MS).
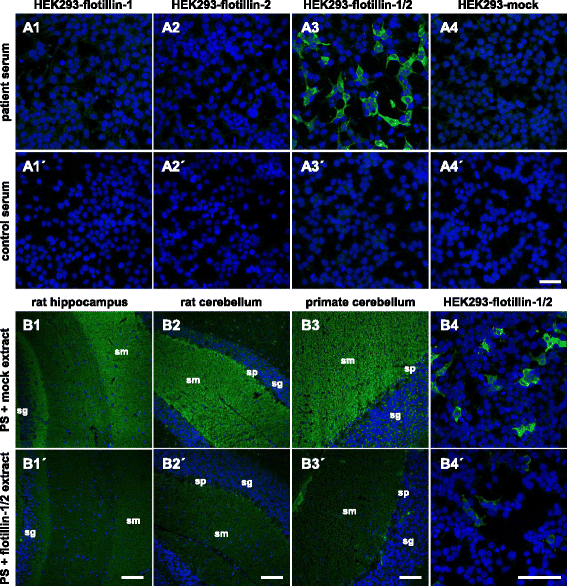
Methods: The target antigen was identified by histo-immunoprecipitation using the patients' sera and cryosections of rat or pig cerebellum combined with mass spectrometrical analysis. Correct identification was ascertained by indirect immunofluorescence and neutralization tests using the target antigens recombinantly expressed in HEK293 cells.
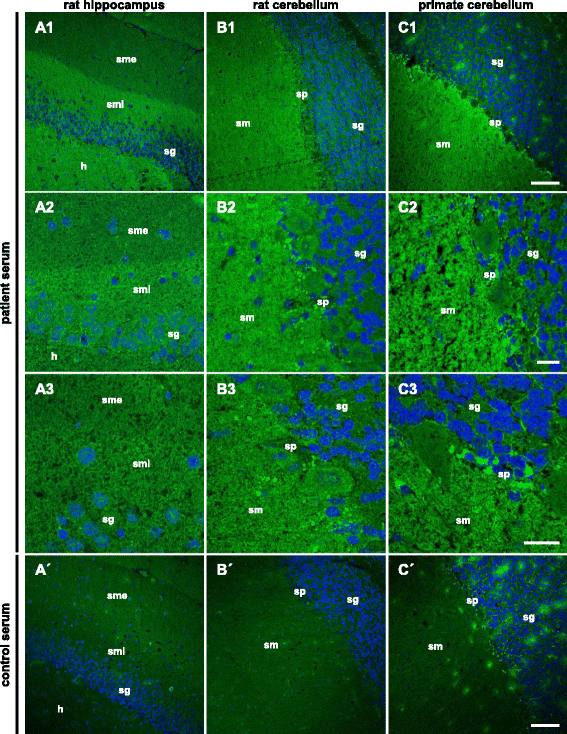
Results: Serum and CSF of the index patient produced a fine-granular IgG indirect immunofluorescence staining of the hippocampal and cerebellar molecular layers. Flotillin-1 and flotillin-2 were identified as target autoantigens. They also reacted with recombinant human flotillin-1/2 co-expressed in HEK293 cells, but not with the individual flotillins in fixed- and live-cell assays. Moreover, neutralization using flotillin-1/2, but not the single flotillins, abolished the tissue reactivity of patient serum. Screening of 521 patients, for whom anti-aquaporin-4 testing was requested and negative, revealed 8 additional patients with anti-flotillin-1/2 autoantibodies. All eight were negative for anti-MOG. Six patients ex post fulfilled the revised McDonald criteria for MS. Vice versa, screening of 538 MS sera revealed anti-flotillin-1/2 autoantibodies in eight patients. The autoantibodies were not found in a cohort of 67 patients with other neural autoantibody-associated syndromes and in 444 healthy blood donors.
Conclusions: Autoantibodies against the flotillin-1/2 heterocomplex, a peripheral membrane protein that is involved in axon outgrowth and regeneration of the optic nerve, are present in 1-2% of patients with bona fide MS.
Keywords: Autoantibodies; Flotillin; Histo-immunoprecipitation; Multiple sclerosis; Optic neuritis; Reggie.
Figures

References
-
- Probst C, Saschenbrecker S, Stöcker W, Komorowski L. Anti-neuronal autoantibodies: current diagnostic challenges. Mult Scler Rel Dis. 2014;3:304–320. - PubMed
-
- Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura A, Ohta K, Iizuka T, Guzman M, Graus F, Moss SJ, Balice-Gordon R, Dalmau J. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67-76. - PMC - PubMed
-
- Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

