MyD88-dependent pro-interleukin-1β induction in dendritic cells exposed to food-grade synthetic amorphous silica
- PMID: 28645296
- PMCID: PMC5481969
- DOI: 10.1186/s12989-017-0202-8
MyD88-dependent pro-interleukin-1β induction in dendritic cells exposed to food-grade synthetic amorphous silica
Abstract
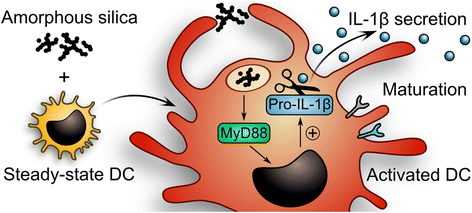
Background: Dendritic cells (DCs) are specialized first-line sensors of foreign materials invading the organism. These sentinel cells rely on pattern recognition receptors such as Nod-like or Toll-like receptors (TLRs) to launch immune reactions against pathogens, but also to mediate tolerance to self-antigens and, in the intestinal milieu, to nutrients and commensals. Since inappropriate DC activation contributes to inflammatory diseases and immunopathologies, a key question in the evaluation of orally ingested nanomaterials is whether their contact with DCs in the intestinal mucosa disrupts this delicate homeostatic balance between pathogen defense and tolerance. Here, we generated steady-state DCs by incubating hematopoietic progenitors with feline McDonough sarcoma-like tyrosine kinase 3 ligand (Flt3L) and used the resulting immature DCs to test potential biological responses against food-grade synthetic amorphous silica (SAS) representing a common nanomaterial generally thought to be safe.
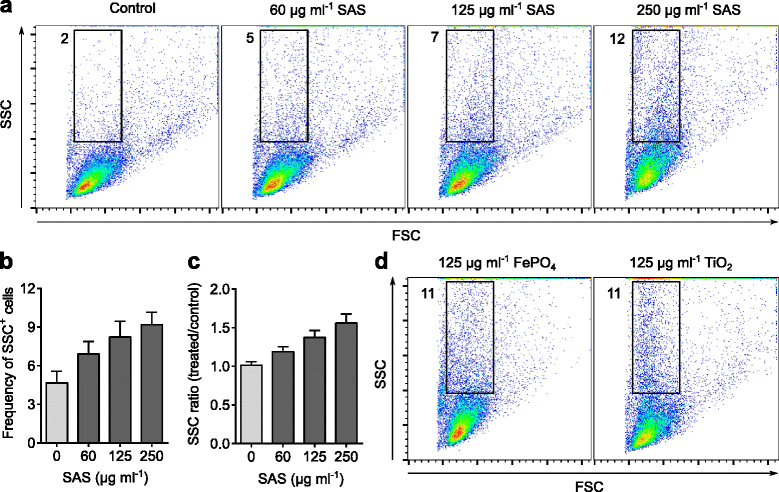
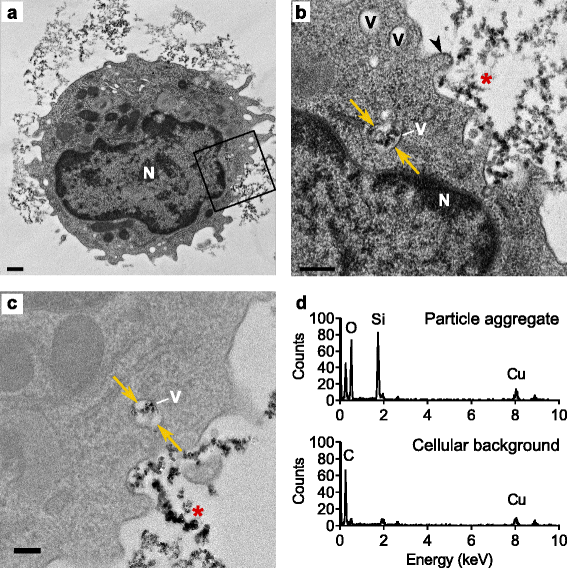
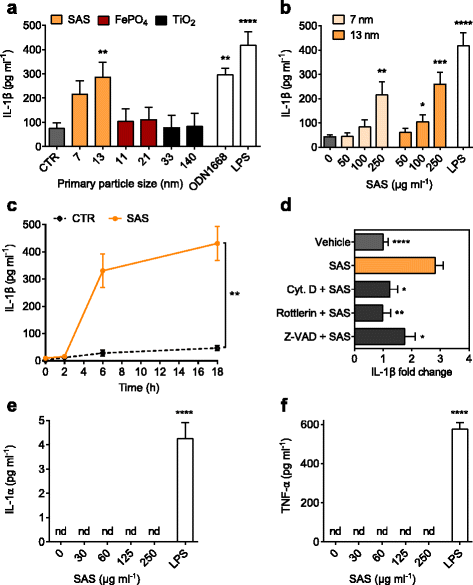
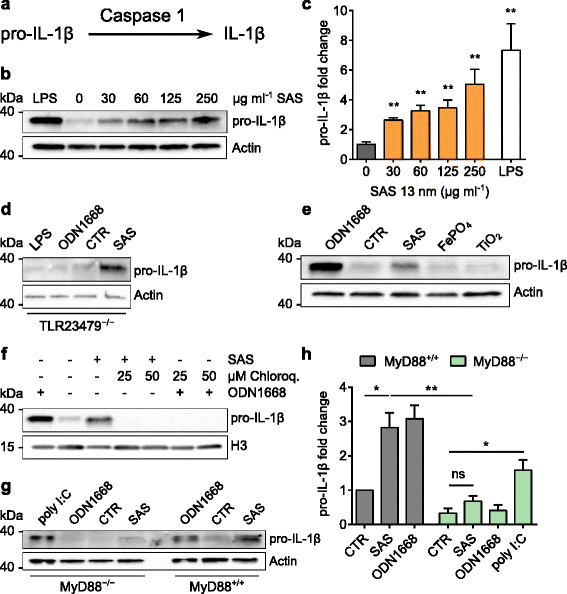
Results: Interaction of immature and unprimed DCs with food-grade SAS particles and their internalization by endocytic uptake fails to elicit cytotoxicity and the release of interleukin (IL)-1α or tumor necrosis factor-α, which were identified as master regulators of acute inflammation in lung-related studies. However, the display of maturation markers on the cell surface shows that SAS particles activate completely immature DCs. Also, the endocytic uptake of SAS particles into these steady-state DCs leads to induction of the pro-IL-1β precursor, subsequently cleaved by the inflammasome to secrete mature IL-1β. In contrast, neither pro-IL-1β induction nor mature IL-1β secretion occurs upon internalization of TiO2 or FePO4 nanoparticles. The pro-IL-1β induction is suppressed by pharmacologic inhibitors of endosomal TLR activation or by genetic ablation of MyD88, a downstream adapter of TLR pathways, indicating that endosomal pattern recognition is responsible for the observed cytokine response to food-grade SAS particles.
Conclusions: Our results unexpectedly show that food-grade SAS particles are able to directly initiate the endosomal MyD88-dependent pathogen pattern recognition and signaling pathway in steady-state DCs. The ensuing activation of immature DCs with de novo induction of pro-IL-1β implies that the currently massive use of SAS particles as food additive should be reconsidered.
Keywords: E 551; Food additive; Food toxicology; Gut-associated lymphoid tissue; Inflammatory bowel disease; Nanomaterial; Silicon dioxide; Synthetic amorphous silica.
Figures

Similar articles
-
TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo.J Immunol. 2013 Jan 1;190(1):334-9. doi: 10.4049/jimmunol.1202737. Epub 2012 Dec 7. J Immunol. 2013. PMID: 23225887 Free PMC article.
-
Synthetic Amorphous Silica Nanoparticles Promote Human Dendritic Cell Maturation and CD4+ T-Lymphocyte Activation.Toxicol Sci. 2021 Dec 28;185(1):105-116. doi: 10.1093/toxsci/kfab120. Toxicol Sci. 2021. PMID: 34633463
-
MyD88 Signaling Regulates Steady-State Migration of Intestinal CD103+ Dendritic Cells Independently of TNF-α and the Gut Microbiota.J Immunol. 2015 Sep 15;195(6):2888-99. doi: 10.4049/jimmunol.1500210. Epub 2015 Aug 10. J Immunol. 2015. PMID: 26259586
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Inflammasome in Dendritic Cells Immunobiology: Implications to Diseases and Therapeutic Strategies.Curr Drug Targets. 2017;18(9):1003-1018. doi: 10.2174/1389450117666160921144830. Curr Drug Targets. 2017. PMID: 27660056 Review.
Cited by
-
Critical review of the safety assessment of titanium dioxide additives in food.J Nanobiotechnology. 2018 Jun 1;16(1):51. doi: 10.1186/s12951-018-0376-8. J Nanobiotechnology. 2018. PMID: 29859103 Free PMC article. Review.
-
Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: potential consequences for host health.Part Fibre Toxicol. 2020 Jun 1;17(1):19. doi: 10.1186/s12989-020-00349-z. Part Fibre Toxicol. 2020. PMID: 32487227 Free PMC article. Review.
-
Pyrogenic and Precipitated Amorphous Silica Nanoparticles Differentially Affect Cell Responses to LPS in Human Macrophages.Nanomaterials (Basel). 2020 Jul 18;10(7):1395. doi: 10.3390/nano10071395. Nanomaterials (Basel). 2020. PMID: 32708373 Free PMC article.
-
Silica Nanoparticles Provoke Cell Death Independent of p53 and BAX in Human Colon Cancer Cells.Nanomaterials (Basel). 2019 Aug 16;9(8):1172. doi: 10.3390/nano9081172. Nanomaterials (Basel). 2019. PMID: 31426331 Free PMC article.
-
The TLR4/NFκB-Dependent Inflammatory Response Activated by LPS Is Inhibited in Human Macrophages Pre-Exposed to Amorphous Silica Nanoparticles.Nanomaterials (Basel). 2022 Jul 5;12(13):2307. doi: 10.3390/nano12132307. Nanomaterials (Basel). 2022. PMID: 35808143 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

