Smooth muscle cells of human veins show an increased response to injury at valve sites
- PMID: 28647196
- PMCID: PMC5740028
- DOI: 10.1016/j.jvs.2017.03.447
Smooth muscle cells of human veins show an increased response to injury at valve sites
Abstract
Objective: Venous valves are essential but are prone to injury, thrombosis, and fibrosis. We compared the behavior and gene expression of smooth muscle cells (SMCs) in the valve sinus vs nonvalve sites to elucidate biologic differences associated with vein valves.
Methods: Tissue explants of fresh human saphenous veins were prepared, and the migration of SMCs from explants of valve sinus vs nonvalve sinus areas was measured. Proliferation and death of SMCs were determined by staining for Ki67 and terminal deoxynucleotidyl transferase dUTP nick end labeling. Proliferation and migration of passaged valve vs nonvalve SMCs were determined by cell counts and using microchemotaxis chambers. Global gene expression in valve vs nonvalve intima-media was determined by RNA sequencing.
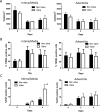
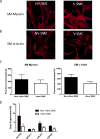
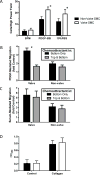
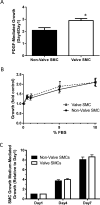
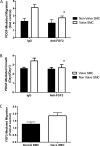
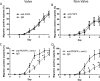
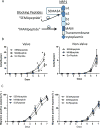
Results: Valve SMCs demonstrated greater proliferation in tissue explants compared with nonvalve SMCs (19.3% ± 5.4% vs 6.8% ± 2.0% Ki67-positive nuclei at 4 days, respectively; mean ± standard error of the mean, five veins; P < .05). This was also true for migration (18.2 ± 2.7 vs 7.5 ± 3.0 migrated SMCs/explant at 6 days, respectively; 24 veins, 15 explants/vein; P < .0001). Cell death was not different (39.6% ± 16.1% vs 41.5% ± 16.0% terminal deoxynucleotidyl transferase dUTP nick end labeling-positive cells, respectively, at 4 days, five veins). Cultured valve SMCs also proliferated faster than nonvalve SMCs in response to platelet-derived growth factor subunit BB (2.9 ± 0.2-fold vs 2.1 ± 0.2-fold of control, respectively; P < .001; n = 5 pairs of cells). This was also true for migration (6.5 ± 1.2-fold vs 4.4 ± 0.8-fold of control, respectively; P < .001; n = 7 pairs of cells). Blockade of fibroblast growth factor 2 (FGF2) inhibited the increased responses of valve SMCs but had no effect on nonvalve SMCs. Exogenous FGF2 increased migration of valve but not of nonvalve SMCs. Unlike in the isolated, cultured cells, blockade of FGF2 in the tissue explants did not block migration of valve or nonvalve SMCs from the explants. Thirty-seven genes were differentially expressed by valve compared with nonvalve intimal-medial tissue (11 veins). Peptide-mediated inhibition of SEMA3A, one of the differentially expressed genes, increased the number of migrated SMCs of valve but not of nonvalve explants.
Conclusions: Valve compared with nonvalve SMCs have greater rates of migration and proliferation, which may in part explain the propensity for pathologic lesion formation in valves. Whereas FGF2 mediates these effects in cultured SMCs, the mediators of these stimulatory effects in the valve wall tissue remain unclear but may be among the differentially expressed genes discovered in this study. One of these genes, SEMA3A, mediates a valve-specific inhibitory effect on the injury response of valve SMCs.
Copyright © 2017 Society for Vascular Surgery. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Epac1 Deficiency Attenuated Vascular Smooth Muscle Cell Migration and Neointimal Formation.Arterioscler Thromb Vasc Biol. 2015 Dec;35(12):2617-25. doi: 10.1161/ATVBAHA.115.306534. Epub 2015 Oct 1. Arterioscler Thromb Vasc Biol. 2015. PMID: 26427796
-
Surgical marking pen dye inhibits saphenous vein cell proliferation and migration in saphenous vein graft tissue.J Vasc Surg. 2016 Apr;63(4):1044-50. doi: 10.1016/j.jvs.2014.10.017. Epub 2015 Apr 30. J Vasc Surg. 2016. PMID: 25935273 Free PMC article.
-
Inhibitory Effects of PRG4 on Migration and Proliferation of Human Venous Cells.J Surg Res. 2020 Sep;253:53-62. doi: 10.1016/j.jss.2020.03.028. Epub 2020 Apr 19. J Surg Res. 2020. PMID: 32320897
-
[Effects of fibrinogen, fibrin and their degradation products on the behaviour of vascular smooth muscle cells].Nihon Ronen Igakkai Zasshi. 2000 Jun;37(6):458-63. doi: 10.3143/geriatrics.37.458. Nihon Ronen Igakkai Zasshi. 2000. PMID: 10998926 Review. Japanese.
-
Transcription Factors Targeted by miRNAs Regulating Smooth Muscle Cell Growth and Intimal Thickening after Vascular Injury.Int J Mol Sci. 2019 Oct 31;20(21):5445. doi: 10.3390/ijms20215445. Int J Mol Sci. 2019. PMID: 31683712 Free PMC article. Review.
Cited by
-
Saphenous vein valve assessment utilizing upright CT to potentially improve graft assessment for bypass surgery.Sci Rep. 2021 Jun 2;11(1):11602. doi: 10.1038/s41598-021-90998-7. Sci Rep. 2021. PMID: 34078949 Free PMC article.
-
Long-term outcomes of great saphenous vein harvest techniques for infrainguinal arterial bypass in a Medicare-matched registry database.J Vasc Surg. 2024 Oct;80(4):1192-1203.e3. doi: 10.1016/j.jvs.2024.05.036. Epub 2024 Jun 22. J Vasc Surg. 2024. PMID: 38912996
-
Hormonal influence: unraveling the impact of sex hormones on vascular smooth muscle cells.Biol Res. 2024 Sep 4;57(1):61. doi: 10.1186/s40659-024-00542-w. Biol Res. 2024. PMID: 39227995 Free PMC article. Review.
-
Semaphorin-3A protects against neointimal hyperplasia after vascular injury.EBioMedicine. 2019 Jan;39:95-108. doi: 10.1016/j.ebiom.2018.12.023. Epub 2018 Dec 19. EBioMedicine. 2019. PMID: 30579864 Free PMC article.
-
Versican is differentially regulated in the adventitial and medial layers of human vein grafts.PLoS One. 2018 Sep 28;13(9):e0204045. doi: 10.1371/journal.pone.0204045. eCollection 2018. PLoS One. 2018. PMID: 30265729 Free PMC article.
References
-
- Eberhardt RT, Raffetto JD. Chronic Venous Insufficiency. Circulation. 2014;130(4):333–46. - PubMed
-
- Ten Cate-Hoek AJ, Henke PK, Wakefield TW. The post thrombotic syndrome: Ignore it and it will come back to bite you. Blood Rev. 2016;30(2):131–7. - PubMed
-
- Vesti BR, Primozich J, Bergelin RO, Strandness E., Jr Follow-up of valves in saphenous vein bypass grafts with duplex ultrasonography. J Vasc Surg. 2001;33(2):369–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

