Hepatocyte-derived macrophage migration inhibitory factor mediates alcohol-induced liver injury in mice and patients
- PMID: 28647568
- PMCID: PMC5650516
- DOI: 10.1016/j.jhep.2017.06.014
Hepatocyte-derived macrophage migration inhibitory factor mediates alcohol-induced liver injury in mice and patients
Abstract
Background & aims: Macrophage migration inhibitory factor (MIF) is a multi-potent cytokine that contributes to the inflammatory response to injury. MIF is expressed by multiple cell types; however, the cellular source and actions of MIF in alcoholic liver disease (ALD) are not well known. Here we tested the hypothesis that non-myeloid cells, specifically hepatocytes, are an important cellular source of MIF in ALD.
Methods: MIF expression was measured in HuH7 and differentiated THP-1 cells in response to ethanol. Ethanol-induced liver injury was assessed in C57BL/6 (WT) and Mif-/- bone marrow chimeras. MIF was measured in peripheral and suprahepatic serum, as well as visualized by immunohistochemistry in liver biopsies, from patients with alcoholic hepatitis (AH).
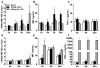
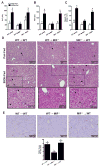
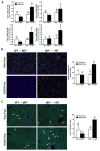
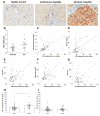
Results: HuH7 hepatocytes, but not THP-1 macrophages, released MIF in response to ethanol challenge in culture. In chimeric mice expressing MIF in non-myeloid cells (Mif-/-→WT), chronic ethanol feeding increased ALT/AST, hepatic steatosis, and expression of cytokine/chemokine mRNA. In contrast, chimeric mice not expressing MIF in non-myeloid cells (WT→Mif-/-) were protected from ethanol-induced liver injury. Immunohistochemical staining of liver biopsies from patients with AH revealed a predominant localization of MIF to hepatocytes. Interestingly, the concentration of MIF in suprahepatic serum, but not peripheral serum, was positively correlated with clinical indicators of disease severity and with an increased risk of mortality in patients with AH.
Conclusions: Taken together, these data provide evidence that hepatocyte-derived MIF is critical in the pathogenesis of ALD in mice and likely contributes to liver injury in patients with AH. Lay summary: Alcoholic liver disease is a major cause of preventable mortality worldwide, and lacks specific pharmacological therapies. Recent studies have recognized that macrophage migration inhibitor factor (MIF) has a critical role in the inflammatory response to liver damage. However, the cells that produce this protein are still unknown. Our present findings reveal that hepatocytes, the main cell type in the liver, are primarily responsible for MIF production in response to alcohol, which promotes liver injury. Our study suggests that drugs inhibiting MIF production could be beneficial in treating patients with liver disease due to excessive alcohol consumption.
Keywords: Alcoholic liver disease; Hepatocytes; Inflammation; Innate immune system; MIF; Translational research.
Copyright © 2017 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Novel Role of Macrophage Migration Inhibitory Factor in Upstream Control of the Unfolded Protein Response After Ethanol Feeding in Mice.Alcohol Clin Exp Res. 2019 Jul;43(7):1439-1451. doi: 10.1111/acer.14065. Epub 2019 May 14. Alcohol Clin Exp Res. 2019. PMID: 31009094 Free PMC article.
-
Macrophage migration inhibitory factor contributes to ethanol-induced liver injury by mediating cell injury, steatohepatitis, and steatosis.Hepatology. 2013 May;57(5):1980-91. doi: 10.1002/hep.26169. Epub 2013 Jan 18. Hepatology. 2013. PMID: 23174952 Free PMC article.
-
Role of MIF in coordinated expression of hepatic chemokines in patients with alcohol-associated hepatitis.JCI Insight. 2021 Jun 8;6(11):e141420. doi: 10.1172/jci.insight.141420. JCI Insight. 2021. PMID: 33945507 Free PMC article.
-
Anti-inflammatory pathways and alcoholic liver disease: role of an adiponectin/interleukin-10/heme oxygenase-1 pathway.World J Gastroenterol. 2010 Mar 21;16(11):1330-6. doi: 10.3748/wjg.v16.i11.1330. World J Gastroenterol. 2010. PMID: 20238399 Free PMC article. Review.
-
Innate immunity and cell death in alcoholic liver disease: role of cytochrome P4502E1.Redox Biol. 2014 Aug 1;2:929-35. doi: 10.1016/j.redox.2014.07.007. eCollection 2014. Redox Biol. 2014. PMID: 25180169 Free PMC article. Review.
Cited by
-
Exercise inhibits JNK pathway activation and lipotoxicity via macrophage migration inhibitory factor in nonalcoholic fatty liver disease.Front Endocrinol (Lausanne). 2022 Sep 6;13:961231. doi: 10.3389/fendo.2022.961231. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36147562 Free PMC article.
-
Unexpected Pro-Fibrotic Effect of MIF in Non-Alcoholic Steatohepatitis Is Linked to a Shift in NKT Cell Populations.Cells. 2021 Jan 28;10(2):252. doi: 10.3390/cells10020252. Cells. 2021. PMID: 33525493 Free PMC article.
-
Inflammation and immunity in liver homeostasis and disease: a nexus of hepatocytes, nonparenchymal cells and immune cells.Cell Mol Immunol. 2025 Jul 1. doi: 10.1038/s41423-025-01313-7. Online ahead of print. Cell Mol Immunol. 2025. PMID: 40595432 Review.
-
Macrophage migration inhibitory factor in acute kidneyinjury.Front Physiol. 2022 Sep 2;13:945827. doi: 10.3389/fphys.2022.945827. eCollection 2022. Front Physiol. 2022. PMID: 36117692 Free PMC article. Review.
-
Could protein content of Urinary Extracellular Vesicles be useful to detect Cirrhosis in Alcoholic Liver Disease?Int J Biol Sci. 2021 May 5;17(8):1864-1877. doi: 10.7150/ijbs.59725. eCollection 2021. Int J Biol Sci. 2021. PMID: 34131392 Free PMC article.
References
-
- Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65:278–90. - PubMed
-
- Barnes MA, McMullen MR, Roychowdhury S, Pisano SG, Liu X, Stavitsky AB, et al. Macrophage migration inhibitory factor contributes to ethanol-induced liver injury by mediating cell injury, steatohepatitis, and steatosis. Hepatol Baltim Md. 2013;57:1980–91. doi: 10.1002/hep.26169. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

