The Warburg Effect and Mass Spectrometry-based Proteomic Analysis
- PMID: 28647695
- PMCID: PMC5572299
- DOI: 10.21873/cgp.20032
The Warburg Effect and Mass Spectrometry-based Proteomic Analysis
Abstract
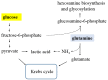
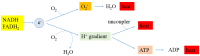
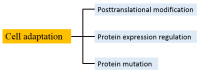
Compared to normal cells, cancer cells have a unique metabolism by performing lactic acid fermentation in the presence of oxygen, also known as the Warburg effect. Researchers have proposed several hypotheses to elucidate the phenomenon, but the mechanism is still an enigma. In this review, we discuss three typical models, such as "damaged mitochondria", "adaptation to hypoxia", and "cell proliferation requirement", as well as contributions from mass spectrometry analysis toward our understanding of the Warburg effect. Mass spectrometry analysis supports the "adaptation to hypoxia" model that cancer cells are using quasi-anaerobic fermentation to reduce oxygen consumption in vivo. We further propose that hypoxia is an early event and it plays a crucial role in carcinoma initiation and development.
Keywords: Cancer metabolism; Warburg effect; mass spectrometry; review.
Copyright© 2017, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Figures
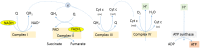
Similar articles
-
Cancer metabolism and mass spectrometry-based proteomics.Cancer Lett. 2015 Jan 28;356(2 Pt A):176-83. doi: 10.1016/j.canlet.2013.11.003. Epub 2013 Nov 18. Cancer Lett. 2015. PMID: 24262660 Review.
-
Cancer metabolism: what we can learn from proteomic analysis by mass spectrometry.Cancer Genomics Proteomics. 2012 Nov;9(6):373-81. Cancer Genomics Proteomics. 2012. PMID: 23162076 Free PMC article. Review.
-
Comparative iTRAQ-Based Quantitative Proteomic Analysis of Pelteobagrus vachelli Liver under Acute Hypoxia: Implications in Metabolic Responses.Proteomics. 2017 Sep;17(17-18). doi: 10.1002/pmic.201700140. Epub 2017 Sep 6. Proteomics. 2017. PMID: 28771929
-
Progress in Mass Spectrometry-Based Proteomics in Hypoxia-Related Diseases and High-Altitude Medicine.OMICS. 2017 Jun;21(6):305-313. doi: 10.1089/omi.2016.0187. Epub 2017 May 9. OMICS. 2017. PMID: 28486083
-
Overflow metabolism in Escherichia coli results from efficient proteome allocation.Nature. 2015 Dec 3;528(7580):99-104. doi: 10.1038/nature15765. Nature. 2015. PMID: 26632588 Free PMC article.
Cited by
-
Dichloroacetate enhances the antitumor efficacy of chemotherapeutic agents via inhibiting autophagy in non-small-cell lung cancer.Cancer Manag Res. 2018 May 16;10:1231-1241. doi: 10.2147/CMAR.S156530. eCollection 2018. Cancer Manag Res. 2018. PMID: 29844702 Free PMC article.
-
Integrated analysis of transcription factors and targets co-expression profiles reveals reduced correlation between transcription factors and target genes in cancer.Funct Integr Genomics. 2019 Jan;19(1):191-204. doi: 10.1007/s10142-018-0636-6. Epub 2018 Sep 24. Funct Integr Genomics. 2019. PMID: 30251028
-
Mitoproteomics: Tackling Mitochondrial Dysfunction in Human Disease.Oxid Med Cell Longev. 2018 Nov 8;2018:1435934. doi: 10.1155/2018/1435934. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30533169 Free PMC article. Review.
-
Regulation of Tissue Growth by the Mammalian Hippo Signaling Pathway.Front Physiol. 2017 Nov 24;8:942. doi: 10.3389/fphys.2017.00942. eCollection 2017. Front Physiol. 2017. PMID: 29225579 Free PMC article. Review.
-
2-Methoxyestradiol Reverses the Pro-Carcinogenic Effect of L-Lactate in Osteosarcoma 143B Cells.Cancer Genomics Proteomics. 2017 Nov-Dec;14(6):483-493. doi: 10.21873/cgp.20058. Cancer Genomics Proteomics. 2017. PMID: 29109098 Free PMC article. Review.
References
-
- Stryer L. Biochemistry. W. H. Freeman and Company. 1995
-
- Yoshikawa S, Muramoto K, Shinzawa-Itoh K, Aoyama H, Tsukihara T, Shimokata K, Katayama Y, Shimada H. Proton pumping mechanism of bovine heart cytochrome c oxidase. Biochim Biophys Acta. 2006;1757:1110–1116. - PubMed
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
