Increased Expression of Follistatin in Breast Cancer Reduces Invasiveness and Clinically Correlates with Better Survival
- PMID: 28647698
- PMCID: PMC5572302
- DOI: 10.21873/cgp.20035
Increased Expression of Follistatin in Breast Cancer Reduces Invasiveness and Clinically Correlates with Better Survival
Abstract
Background/aim: Activin and its antagonist follistatin (FST) have been implicated in several solid tumours. This study investigated the role of FST in breast cancer.
Materials and methods: FST expression was examined using reverse transcription polymerase chain reaction (RT-PCR), real-time quantitative polymerase chain reaction (qPCR) and immunohistochemistry in a cohort of breast cancer samples. Expression was correlated to pathological and prognostic parameters in our patient cohort. FST was overexpressed in MCF-7 cells and assays for growth and invasion were performed.
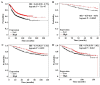
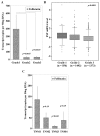
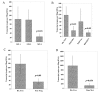
Results: FST is expressed in breast tissue, in the cytoplasm of mammary epithelial cells. Expression was decreased in breast cancer tissue in comparison to normal mammary tissue. Over-expression of FST in vitro led to significantly increased growth rate and reduced invasion. Higher FST associates with lower-grade tumours and better survival.
Conclusion: Our results suggest a role for FST as a suppressor of invasion and metastasis in breast cancer.
Keywords: Follistatin; breast cancer; survival.
Copyright© 2017, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Figures



References
-
- Nolan K, Thompson TB. Bmp and bmp regulation: Structure and function. In: Bone morphogenetic proteins: Systems biology regulators. Vukicevic S and Sampath KT (eds.) Springer International Publishing. 2017:pp. 73–111.
-
- Krneta J, Kroll J, Alves F, Prahst C, Sananbenesi F, Dullin C, Kimmina S, Phillips DJ, Augustin HG. Dissociation of angiogenesis and tumorigenesis in follistatin- and activin-expressing tumors. Cancer Res. 2006;66(11):5686–5695. - PubMed
-
- Harrison CA, Gray PC, Vale WW, Robertson DM. Antagonists of activin signaling: Mechanisms and potential biological applications. Trends Endocrinol Metab. 2005;16(2):73–78. - PubMed
-
- Sugino K, Kurosawa N, Nakamura T, Takio K, Shimasaki S, Ling N, Titani K, Sugino H. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J Biol Chem. 1993;268(21):15579–15587. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
