Impact of titin strain on the cardiac slow force response
- PMID: 28648628
- PMCID: PMC5716905
- DOI: 10.1016/j.pbiomolbio.2017.06.009
Impact of titin strain on the cardiac slow force response
Abstract
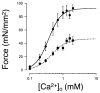
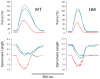
Stretch of myocardium, such as occurs upon increased filling of the cardiac chamber, induces two distinct responses: an immediate increase in twitch force followed by a slower increase in twitch force that develops over the course of several minutes. The immediate response is due, in part, to modulation of myofilament Ca2+ sensitivity by sarcomere length (SL). The slowly developing force response, termed the Slow Force Response (SFR), is caused by a slowly developing increase in intracellular Ca2+ upon sustained stretch. A blunted immediate force response was recently reported for myocardium isolated from homozygous giant titin mutant rats (HM) compared to muscle from wild-type littermates (WT). Here, we examined the impact of titin isoform on the SFR. Right ventricular trabeculae were isolated and mounted in an experimental chamber. SL was measured by laser diffraction. The SFR was recorded in response to a 0.2 μm SL stretch in the presence of [Ca2+]o = 0.4 mM, a bathing concentration reflecting ∼50% of maximum twitch force development at 25 °C. Presence of the giant titin isoform (HM) was associated with a significant reduction in diastolic passive force upon stretch, and ∼50% reduction of the magnitude of the SFR; the rate of SFR development was unaffected. The sustained SL stretch was identical in both muscle groups. Therefore, our data suggest that cytoskeletal strain may underlie directly the cellular mechanisms that lead to the increased intracellular [Ca2+]i that causes the SFR, possibly by involving cardiac myocyte integrin signaling pathways.
Keywords: Isolated cardiac trabeculae; Passive force; Rat; Stretch; Twitch force.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
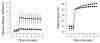

References
-
- Cingolani HE, Ennis IL, Aiello EA, Pérez NG. Role of autocrine/paracrine mechanisms in response to myocardial strain. Pflugers Arch. 2011;462:29–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

