Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways
- PMID: 28648659
- PMCID: PMC5839326
- DOI: 10.1016/j.cell.2017.05.034
Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways
Abstract
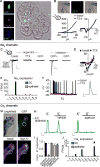
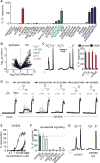
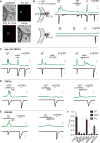
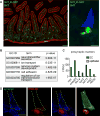
Dietary, microbial, and inflammatory factors modulate the gut-brain axis and influence physiological processes ranging from metabolism to cognition. The gut epithelium is a principal site for detecting such agents, but precisely how it communicates with neural elements is poorly understood. Serotonergic enterochromaffin (EC) cells are proposed to fulfill this role by acting as chemosensors, but understanding how these rare and unique cell types transduce chemosensory information to the nervous system has been hampered by their paucity and inaccessibility to single-cell measurements. Here, we circumvent this limitation by exploiting cultured intestinal organoids together with single-cell measurements to elucidate intrinsic biophysical, pharmacological, and genetic properties of EC cells. We show that EC cells express specific chemosensory receptors, are electrically excitable, and modulate serotonin-sensitive primary afferent nerve fibers via synaptic connections, enabling them to detect and transduce environmental, metabolic, and homeostatic information from the gut directly to the nervous system.
Keywords: chemosensation; enterochromaffin cell; gastrointestinal physiology; inflammatory bowel disease; intestinal organoid; microbial metabolites; neurogastroenterology; nociception; sensory transduction; visceral pain.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Comment in
-
How the Gut Feels, Smells, and Talks.Cell. 2017 Jun 29;170(1):10-11. doi: 10.1016/j.cell.2017.06.023. Cell. 2017. PMID: 28666112
-
Neurogastroenterology: Tasting the gut - enterochromaffin cells are chemosensitive and modulate sensory neurons.Nat Rev Gastroenterol Hepatol. 2017 Aug;14(8):449. doi: 10.1038/nrgastro.2017.91. Epub 2017 Jul 5. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28676710 No abstract available.
-
Gut-brain communication: Making contact.Nat Rev Neurosci. 2017 Aug;18(8):452. doi: 10.1038/nrn.2017.89. Epub 2017 Jul 13. Nat Rev Neurosci. 2017. PMID: 28720820 No abstract available.
References
-
- Aiken KD, Roth KA. Temporal differentiation and migration of substance P, serotonin, and secretin immunoreactive enteroendocrine cells in the mouse proximal small intestine. Dev Dyn. 1992;194:303–310. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
-
- Bertrand PP, Hu X, Mach J, Bertrand RL. Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. Am J Physiol-Gastr L. 2008;295:G1228–G1236. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

