EphA2 and ephrin-A5 are not a receptor-ligand pair in the ocular lens
- PMID: 28648759
- PMCID: PMC5554726
- DOI: 10.1016/j.exer.2017.06.016
EphA2 and ephrin-A5 are not a receptor-ligand pair in the ocular lens
Abstract
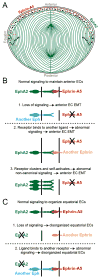
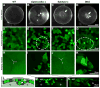
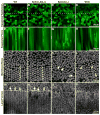
Eph-ephrin bidirectional signaling is essential for eye lens transparency in humans and mice. Our previous studies in mouse lenses demonstrate that ephrin-A5 is mainly expressed in the anterior epithelium, where it is required for maintaining the anterior epithelial monolayer. In contrast, EphA2 is localized in equatorial epithelial and fiber cells where it is essential for equatorial epithelial and fiber cell organization and hexagonal cell shape. Immunostaining of lens epithelial and fiber cells reveals that EphA2 and ephrin-A5 are also co-expressed in anterior fiber cell tips, equatorial epithelial cells and newly formed lens fibers, although they are not precisely colocalized. Due to this complex expression pattern and the promiscuous interactions between Eph receptors and ephrin ligands, as well as their complex bidirectional signaling pathways, cataracts in ephrin-A5(-/-) or EphA2(-/-) lenses may arise from loss of function or abnormal signaling mechanisms. To test whether abnormal signaling mechanisms may play a role in cataractogenesis in ephrin-A5(-/-) or EphA2(-/-) lenses, we generated EphA2 and ephrin-A5 double knockout (DKO) mice. We compared the phenotypes of EphA2(-/-) and ephrin-A5(-/-) lenses to that of DKO lenses. DKO lenses displayed an additive lens phenotype that was not significantly different from the two single KO lens phenotypes. Similar to ephrin-A5(-/-) lenses, DKO lenses had abnormal anterior epithelial cells leading to a large mass of epithelial cells that invade into the underlying fiber cell layer, directly resulting in anterior cataracts in ephrin-A5(-/-) and DKO lenses. Yet, similar to EphA2(-/-) lenses, DKO lenses also had abnormal packing of equatorial epithelial cells with disorganized meridional rows, lack of a lens fulcrum and disrupted fiber cells. The DKO lens phenotype rules out abnormal signaling by EphA2 in ephrin-A5(-/-) lenses or by ephrin-A5 in EphA2(-/-) lenses as possible cataract mechanisms. Thus, these results indicate that EphA2 and ephrin-A5 do not form a lens receptor-ligand pair, and that EphA2 and ephrin-A5 have other binding partners in the lens to help align differentiating equatorial epithelial cells or maintain the anterior epithelium, respectively.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


References
-
- Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365:599–609. - PubMed
-
- Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nature cell biology. 2010;12:1194–1204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

