Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells
- PMID: 28648983
- PMCID: PMC5539982
- DOI: 10.1016/j.cmet.2017.05.016
Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells
Abstract
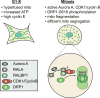
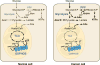
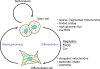
Cancer and stem cells appear to share a common metabolic profile that is characterized by high utilization of glucose through aerobic glycolysis. In the presence of sufficient nutrients, this metabolic strategy provides sufficient cellular ATP while additionally providing important metabolites necessary for the biosynthetic demands of continuous cell proliferation. Recent studies indicate that this metabolic profile is dependent on genes that regulate the fusion and fission of mitochondria. High levels of mitochondrial fission activity are associated with high proliferation and invasiveness in some cancer cells and with self-renewal and resistance to differentiation in some stem cells. These observations reveal new ways in which mitochondria regulate cell physiology, through their effects on metabolism and cell signaling.
Keywords: cancer; induced pluripotential stem cells; metabolism; mitochondria; mitochondrial dynamics; stem cells.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissiere A, Campos Y, Rivera H, de la Aleja JG, Carroccia R, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy 'plus' phenotypes. Brain. 2008;131:338–351. - PubMed
-
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Developmental cell. 2008;14:193–204. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

