Injectable scaffolds: Preparation and application in dental and craniofacial regeneration
- PMID: 28649171
- PMCID: PMC5478172
- DOI: 10.1016/j.mser.2016.11.001
Injectable scaffolds: Preparation and application in dental and craniofacial regeneration
Abstract
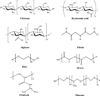
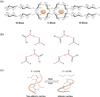
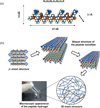
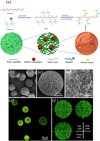
Injectable scaffolds are appealing for tissue regeneration because they offer many advantages over pre-formed scaffolds. This article provides a comprehensive review of the injectable scaffolds currently being investigated for dental and craniofacial tissue regeneration. First, we provide an overview of injectable scaffolding materials, including natural, synthetic, and composite biomaterials. Next, we discuss a variety of characteristic parameters and gelation mechanisms of the injectable scaffolds. The advanced injectable scaffolding systems developed in recent years are then illustrated. Furthermore, we summarize the applications of the injectable scaffolds for the regeneration of dental and craniofacial tissues that include pulp, dentin, periodontal ligament, temporomandibular joint, and alveolar bone. Finally, our perspectives on the injectable scaffolds for dental and craniofacial tissue regeneration are offered as signposts for the future advancement of this field.
Keywords: craniofacial; dental; injectable; scaffold; tissue regeneration.
Figures




References
-
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, Eke PI, Beltran-Aguilar ED, Horowitz AM, Li CH. Vital. Health. Stat. 2007;11:1–92. - PubMed
-
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. J. Dent. Res. 2012;91:914–920. - PubMed
-
- Adell R, Eriksson B, Lekholm U, Branemark PI, Jemt T. Int. J. Oral. Maxillofac. Implants. 1990;5:347–359. - PubMed
-
- Allen KD, Athanasiou KA. Tissue Eng. 2006;12:1183–1196. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
