An Antigen-Presenting and Apoptosis-Inducing Polymer Microparticle Prolongs Alloskin Graft Survival by Selectively and Markedly Depleting Alloreactive CD8+ T Cells
- PMID: 28649247
- PMCID: PMC5465244
- DOI: 10.3389/fimmu.2017.00657
An Antigen-Presenting and Apoptosis-Inducing Polymer Microparticle Prolongs Alloskin Graft Survival by Selectively and Markedly Depleting Alloreactive CD8+ T Cells
Abstract
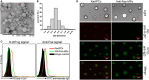
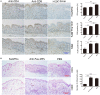
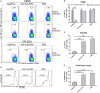
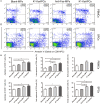
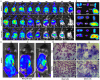
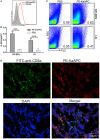
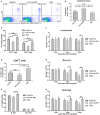
Selectively depleting the pathogenic T cells is a fundamental strategy for the treatment of allograft rejection and autoimmune disease since it retains the overall immune function of host. The concept of killer artificial antigen-presenting cells (KaAPCs) has been developed by co-coupling peptide-major histocompatibility complex (pMHC) multimer and anti-Fas monoclonal antibody (mAb) onto the polymeric microparticles (MPs) to induce the apoptosis of antigen-specific T cells. But little information is available about its in vivo therapeutic potential and mechanism. In this study, polyethylenimine (PEI)-coated poly lactic-co-glycolic acid microparticle (PLGA MP) was fabricated as a cell-sized scaffold to covalently co-couple H-2Kb-Ig dimer and anti-Fas mAb for the generation of alloantigen-presenting and apoptosis-inducing MPs. Intravenous infusions of the biodegradable KaAPCs prolonged the alloskin graft survival for 43 days in a single MHC-mismatched murine model, depleted the most of H-2Kb-alloreactive CD8+ T cells in peripheral blood, spleen, and alloskin graft in an antigen-specific manner and anti-Fas-dependent fashion. The cell-sized KaAPCs circulated throughout vasculature into liver, kidney, spleen, lymph nodes, lung, and heart, but few ones into local allograft at early stage, with a retention time up to 36 h in vivo. They colocalized with CD8+ T cells in secondary lymphoid organs while few ones contacted with CD4+ T cells, B cells, macrophage, and dendritic cells, or internalized by phagocytes. Importantly, the KaAPC treatment did not significantly impair the native T cell repertoire or non-pathogenic immune cells, did not obviously suppress the overall immune function of host, and did not lead to visible organ toxicity. Our results strongly document the high potential of PLGA MP-based KaAPCs as a novel antigen-specific immunotherapy for allograft rejection and autoimmune disorder. The in vivo mechanism of alloinhibition, tissue distribution, and biosafety were also initially characterized, which will facilitate its translational studies from bench to bedside.
Keywords: allograft rejection; alloreactive T cells; biomimetic microparticle; killer artificial antigen-presenting cell; peptide–major histocompatibility complex; poly lactic-co-glycolic acid.
Figures


Similar articles
-
On-target and direct modulation of alloreactive T cells by a nanoparticle carrying MHC alloantigen, regulatory molecules and CD47 in a murine model of alloskin transplantation.Drug Deliv. 2018 Nov;25(1):703-715. doi: 10.1080/10717544.2018.1447049. Drug Deliv. 2018. PMID: 29508634 Free PMC article.
-
Killer artificial antigen-presenting cells deplete alloantigen-specific T cells in a murine model of alloskin transplantation.Immunol Lett. 2011 Aug 30;138(2):144-55. doi: 10.1016/j.imlet.2011.04.002. Epub 2011 Apr 12. Immunol Lett. 2011. PMID: 21513739
-
A biodegradable killer microparticle to selectively deplete antigen-specific T cells in vitro and in vivo.Oncotarget. 2016 Mar 15;7(11):12176-90. doi: 10.18632/oncotarget.7519. Oncotarget. 2016. PMID: 26910923 Free PMC article.
-
[MHC tetramers: tracking specific immunity].Acta Med Croatica. 2003;57(4):255-9. Acta Med Croatica. 2003. PMID: 14639858 Review. Croatian.
-
[The immune response to kidney allograft. Part I: the role of MHC antigens, antigen-presenting cells, and lymphocytes in alloantigen recognition; two-signal activation of T cells].Postepy Hig Med Dosw (Online). 2007;61:13-20. Postepy Hig Med Dosw (Online). 2007. PMID: 17245313 Review. Polish.
Cited by
-
Implantable Immunosuppressant Delivery to Prevent Rejection in Transplantation.Int J Mol Sci. 2022 Jan 29;23(3):1592. doi: 10.3390/ijms23031592. Int J Mol Sci. 2022. PMID: 35163514 Free PMC article. Review.
-
On-target and direct modulation of alloreactive T cells by a nanoparticle carrying MHC alloantigen, regulatory molecules and CD47 in a murine model of alloskin transplantation.Drug Deliv. 2018 Nov;25(1):703-715. doi: 10.1080/10717544.2018.1447049. Drug Deliv. 2018. PMID: 29508634 Free PMC article.
-
Chemically Engineered Immune Cell-Derived Microrobots and Biomimetic Nanoparticles: Emerging Biodiagnostic and Therapeutic Tools.Adv Sci (Weinh). 2021 Mar 1;8(8):2002499. doi: 10.1002/advs.202002499. eCollection 2021 Apr. Adv Sci (Weinh). 2021. PMID: 33898169 Free PMC article. Review.
-
Immunomodulatory effect of PLGA-encapsulated mesenchymal stem cells-derived exosomes for the treatment of allergic rhinitis.Front Immunol. 2024 Jul 8;15:1429442. doi: 10.3389/fimmu.2024.1429442. eCollection 2024. Front Immunol. 2024. PMID: 39040099 Free PMC article.
-
Tolerogenic dendritic cells in organ transplantation.Transpl Int. 2020 Feb;33(2):113-127. doi: 10.1111/tri.13504. Epub 2019 Oct 29. Transpl Int. 2020. PMID: 31472079 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

