Muscle development in the shark Scyliorhinus canicula: implications for the evolution of the gnathostome head and paired appendage musculature
- PMID: 28649268
- PMCID: PMC5480186
- DOI: 10.1186/s12983-017-0216-y
Muscle development in the shark Scyliorhinus canicula: implications for the evolution of the gnathostome head and paired appendage musculature
Abstract
Background: The origin of jawed vertebrates was marked by profound reconfigurations of the skeleton and muscles of the head and by the acquisition of two sets of paired appendages. Extant cartilaginous fish retained numerous plesiomorphic characters of jawed vertebrates, which include several aspects of their musculature. Therefore, myogenic studies on sharks are essential in yielding clues on the developmental processes involved in the origin of the muscular anatomy.
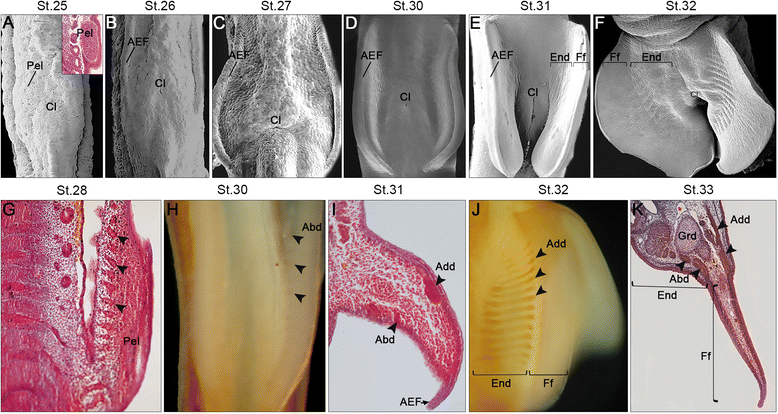
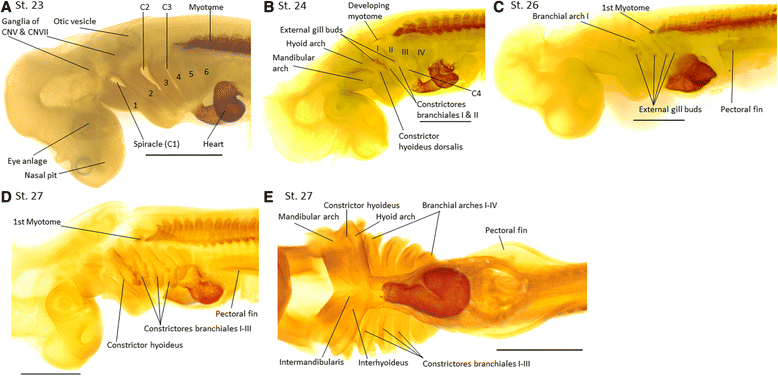
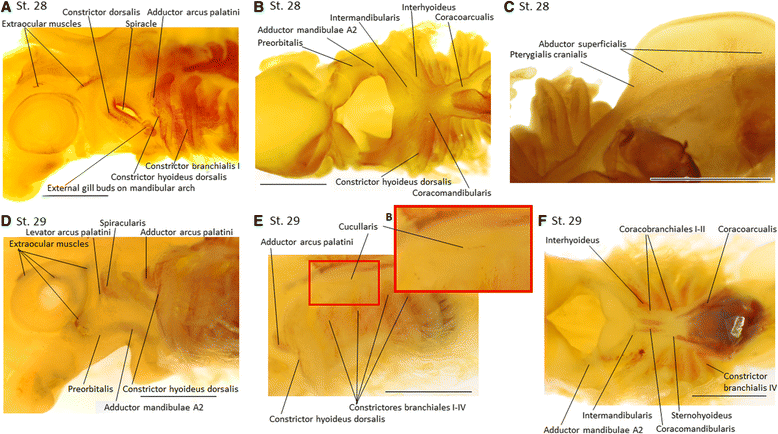
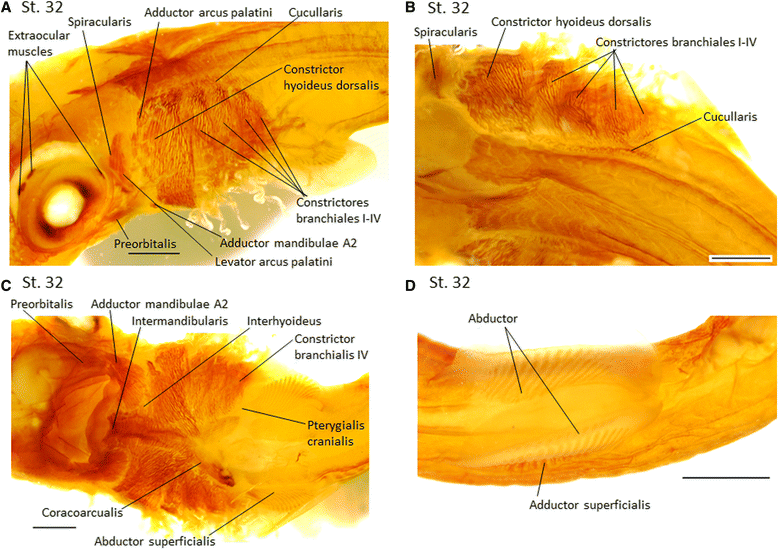
Results: Here we provide a detailed description of the development of specific muscular units integrating the cephalic and appendicular musculature of the shark model, Scyliorhinus canicula. In addition, we analyze the muscle development across gnathostomes by comparing the developmental onset of muscle groups in distinct taxa. Our data reveal that appendicular myogenesis occurs earlier in the pectoral than in the pelvic appendages. Additionally, the pectoral musculature includes muscles that have their primordial developmental origin in the head. This culminates in a tight muscular connection between the pectoral girdle and the cranium, which founds no parallel in the pelvic fins. Moreover, we identified a lateral to ventral pattern of formation of the cephalic muscles, that has been equally documented in osteichthyans but, in contrast with these gnathostomes, the hyoid muscles develop earlier than mandibular muscle in S. canicula.
Conclusion: Our analyses reveal considerable differences in the formation of the pectoral and pelvic musculatures in S. canicula, reinforcing the idea that head tissues have contributed to the formation of the pectoral appendages in the common ancestor of extant gnathostomes. In addition, temporal differences in the formation of some cranial muscles between chondrichthyans and osteichthyans might support the hypothesis that the similarity between the musculature of the mandibular arch and of the other pharyngeal arches represents a derived feature of jawed vertebrates.
Keywords: Cranial; Cucullaris; Fin; Head; Limb; Muscles; Pectoral; Pelvic; Shark.
Figures


Similar articles
-
Muscles of chondrichthyan paired appendages: comparison with osteichthyans, deconstruction of the fore-hindlimb serial homology dogma, and new insights on the evolution of the vertebrate neck.Anat Rec (Hoboken). 2015 Mar;298(3):513-30. doi: 10.1002/ar.23047. Epub 2014 Sep 17. Anat Rec (Hoboken). 2015. PMID: 25205543
-
Comparative anatomy of zebrafish paired and median fin muscles: basis for functional, developmental, and macroevolutionary studies.J Anat. 2018 Feb;232(2):186-199. doi: 10.1111/joa.12728. Epub 2017 Nov 17. J Anat. 2018. PMID: 29148042 Free PMC article.
-
Development of hypobranchial muscles with special reference to the evolution of the vertebrate neck.Zoological Lett. 2018 Feb 18;4:5. doi: 10.1186/s40851-018-0087-x. eCollection 2018. Zoological Lett. 2018. PMID: 29468087 Free PMC article.
-
Developmental Evolution of Hypaxial Muscles: Insights From Cyclostomes and Chondrichthyans.Front Cell Dev Biol. 2021 Sep 28;9:760366. doi: 10.3389/fcell.2021.760366. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34650989 Free PMC article. Review.
-
Cranial or postcranial-Dual origin of the pectoral appendage of vertebrates combining the fin-fold and gill-arch theories?Dev Dyn. 2020 Oct;249(10):1182-1200. doi: 10.1002/dvdy.192. Epub 2020 May 26. Dev Dyn. 2020. PMID: 32395826 Review.
Cited by
-
Unique morphogenetic signatures define mammalian neck muscles and associated connective tissues.Elife. 2018 Nov 19;7:e40179. doi: 10.7554/eLife.40179. Elife. 2018. PMID: 30451684 Free PMC article.
-
The Functional Significance of Cardiac Looping: Comparative Embryology, Anatomy, and Physiology of the Looped Design of Vertebrate Hearts.J Cardiovasc Dev Dis. 2024 Aug 17;11(8):252. doi: 10.3390/jcdd11080252. J Cardiovasc Dev Dis. 2024. PMID: 39195160 Free PMC article. Review.
-
Novel developmental bases for the evolution of hypobranchial muscles in vertebrates.BMC Biol. 2020 Sep 9;18(1):120. doi: 10.1186/s12915-020-00851-y. BMC Biol. 2020. PMID: 32907560 Free PMC article.
-
The larval chondrocranium and its development in Smilisca phaeota with considerations of patterns characteristic for the chondrocranial development of Lalagobatrachia.Sci Rep. 2024 Aug 26;14(1):19779. doi: 10.1038/s41598-024-70724-9. Sci Rep. 2024. PMID: 39187639 Free PMC article.
-
The evolutionary origins and diversity of the neuromuscular system of paired appendages in batoids.Proc Biol Sci. 2019 Nov 6;286(1914):20191571. doi: 10.1098/rspb.2019.1571. Epub 2019 Oct 30. Proc Biol Sci. 2019. PMID: 31662089 Free PMC article.
References
-
- Janvier P. Early vertebrates: Oxford University Press; 1996.
LinkOut - more resources
Full Text Sources
Other Literature Sources

