Surface topology affects wetting behavior of Bacillus subtilis biofilms
- PMID: 28649412
- PMCID: PMC5460217
- DOI: 10.1038/s41522-017-0018-1
Surface topology affects wetting behavior of Bacillus subtilis biofilms
Abstract
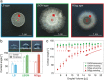
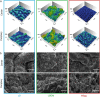
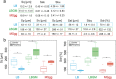
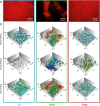
The colonization of surfaces by bacterial biofilms constitutes a huge problem in healthcare and industry. When attempting biofilm inactivation or removal, it is crucial to sufficiently wet the biofilm surface with antibacterial agents; however, certain biofilms efficiently resist wetting, and the origin of this behavior remains to date unclear. Here, we demonstrate that, depending on the growth medium used, the model bacterium Bacillus subtilis can form biofilm colonies with distinct surface properties: we find either hydrophilic or two variants of hydrophobic behavior. We show that those differences in biofilm wetting correlate with distinct surface topologies which, in turn, give rise to different physical wetting regimes known from lotus leaves or rose petals. Forming biofilms with different wetting properties may help bacteria to survive in both arid and humid conditions. Furthermore, converting the surface polarity of a biofilm could facilitate their removal from surfaces by increasing their wettability.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Neinhuis C, Barthlott W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997;79:667–677. doi: 10.1006/anbo.1997.0400. - DOI
-
- Feng L, et al. Super-hydrophobic surfaces: from natural to artificial. Adv. Mater. 2002;14:1857–1860. doi: 10.1002/adma.200290020. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

