Molecular stratification of early breast cancer identifies drug targets to drive stratified medicine
- PMID: 28649643
- PMCID: PMC5445616
- DOI: 10.1038/s41523-016-0003-5
Molecular stratification of early breast cancer identifies drug targets to drive stratified medicine
Abstract
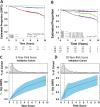
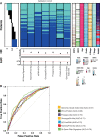
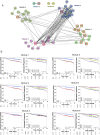
Many women with hormone receptor-positive early breast cancer can be managed effectively with endocrine therapies alone. However, additional systemic chemotherapy treatment is necessary for others. The clinical challenges in managing high-risk women are to identify existing and novel druggable targets, and to identify those who would benefit from these therapies. Therefore, we performed mRNA abundance analysis using the Tamoxifen and Exemestane Adjuvant Multinational (TEAM) trial pathology cohort to identify a signature of residual risk following endocrine therapy and pathways that are potentially druggable. A panel of genes compiled from academic and commercial multiparametric tests as well as genes of importance to breast cancer pathogenesis was used to profile 3825 patients. A signature of 95 genes, including nodal status, was validated to stratify endocrine-treated patients into high-risk and low-risk groups based on distant relapse-free survival (DRFS; Hazard Ratio = 5.05, 95% CI 3.53-7.22, p = 7.51 × 10-19). This risk signature was also found to perform better than current multiparametric tests. When the 95-gene prognostic signature was applied to all patients in the validation cohort, including patients who received adjuvant chemotherapy, the signature remained prognostic (HR = 4.76, 95% CI 3.61-6.28, p = 2.53× 10-28). Functional gene interaction analyses identified six significant modules representing pathways involved in cell cycle control, mitosis and receptor tyrosine signaling; containing a number of genes with existing targeted therapies for use in breast or other malignancies. Thus the identification of high-risk patients using this prognostic signature has the potential to also prioritize patients for treatment with these targeted therapies.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

