κ-Opioid Receptor Activation in Dopamine Neurons Disrupts Behavioral Inhibition
- PMID: 28649993
- PMCID: PMC5729556
- DOI: 10.1038/npp.2017.133
κ-Opioid Receptor Activation in Dopamine Neurons Disrupts Behavioral Inhibition
Abstract
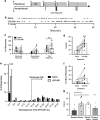
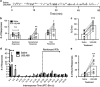
The dynorphin/κ-opioid receptor (KOR) system has been previously implicated in the regulation of cognition, but the neural circuitry and molecular mechanisms underlying KOR-mediated cognitive disruption are unknown. Here, we used an operational test of cognition involving timing and behavioral inhibition and found that systemic KOR activation impairs performance of male and female C57BL/6 mice in the differential reinforcement of low response rate (DRL) task. Systemic KOR antagonism also blocked stress-induced disruptions of DRL performance. KOR activation increased 'bursts' of incorrect responses in the DRL task and increased marble burying, suggesting that the observed disruptions in DRL performance may be attributed to KOR-induced increases in compulsive behavior. Local inactivation of KOR by injection of the long-acting antagonist nor-BNI in the ventral tegmental area (VTA), but not the infralimbic prefrontal cortex (PFC) or dorsal raphe nucleus (DRN), prevented disruption of DRL performance caused by systemic KOR activation. Cre-dependent genetic excision of KOR from dopaminergic, but not serotonergic neurons, also blocked KOR-mediated disruption of DRL performance. At the molecular level, we found that these disruptive effects did not require arrestin-dependent signaling, because neither global deletion of G-protein receptor kinase 3 (GRK3) nor cell-specific deletion of GRK3/arrestin-dependent p38α MAPK from dopamine neurons blocked KOR-mediated DRL disruptions. We then showed that nalfurafine, a clinically available G-biased KOR agonist, could also produce DRL disruptions. Together, these studies demonstrate that KOR activation in VTA dopamine neurons disrupts behavioral inhibition in a GRK3/arrestin-independent manner and suggests that KOR antagonists could be beneficial for decreasing stress-induced compulsive behaviors.
Figures



References
-
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993). Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264: 489–495. - PubMed
-
- Beatty WW (1973). Effects of gonadectomy on sex differences in DRL behavior. Physiol Behav 10: 177–178. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

