Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression
- PMID: 28650342
- PMCID: PMC5531402
- DOI: 10.1172/JCI75005
Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression
Abstract
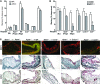
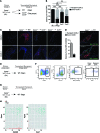
Atherosclerosis is a chronic inflammatory disease, and developing therapies to promote its regression is an important clinical goal. We previously established that atherosclerosis regression is characterized by an overall decrease in plaque macrophages and enrichment in markers of alternatively activated M2 macrophages. We have now investigated the origin and functional requirement for M2 macrophages in regression in normolipidemic mice that received transplants of atherosclerotic aortic segments. We compared plaque regression in WT normolipidemic recipients and those deficient in chemokine receptors necessary to recruit inflammatory Ly6Chi (Ccr2-/- or Cx3cr1-/-) or patrolling Ly6Clo (Ccr5-/-) monocytes. Atherosclerotic plaques transplanted into WT or Ccr5-/- recipients showed reduced macrophage content and increased M2 markers consistent with plaque regression, whereas plaques transplanted into Ccr2-/- or Cx3cr1-/- recipients lacked this regression signature. The requirement of recipient Ly6Chi monocyte recruitment was confirmed in cell trafficking studies. Fate-mapping and single-cell RNA sequencing studies also showed that M2-like macrophages were derived from newly recruited monocytes. Furthermore, we used recipient mice deficient in STAT6 to demonstrate a requirement for this critical component of M2 polarization in atherosclerosis regression. Collectively, these results suggest that continued recruitment of Ly6Chi inflammatory monocytes and their STAT6-dependent polarization to the M2 state are required for resolution of atherosclerotic inflammation and plaque regression.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

