Immature Midbrain Dopaminergic Neurons Derived from Floor-Plate Method Improve Cell Transplantation Therapy Efficacy for Parkinson's Disease
- PMID: 28650520
- PMCID: PMC5689771
- DOI: 10.1002/sctm.16-0470
Immature Midbrain Dopaminergic Neurons Derived from Floor-Plate Method Improve Cell Transplantation Therapy Efficacy for Parkinson's Disease
Abstract
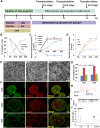
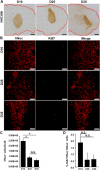
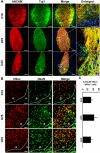
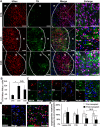
Recent reports have indicated human embryonic stem cells-derived midbrain dopamine (mDA) neurons as proper cell resources for use in Parkinson's disease (PD) therapy. Nevertheless, no detailed and systematic study has been conducted to identify which differentiation stages of mDA cells are most suitable for transplantation in PD therapy. Here, we transplanted three types of mDA cells, DA progenitors (differentiated in vitro for 16 days [D16]), immature DA neurons (D25), and DA neurons (D35), into PD mice and found that all three types of cells showed high viability and strong neuronal differentiation in vivo. Both D25 and D35 cells showed neuronal maturation and differentiation toward TH+ cells and, accordingly, satisfactory behavioral functional recovery. However, transplanted D16 cells were less capable of producing functional recovery. These findings provide a valuable guideline for standardizing the differentiation stage of the transplantable cells used in clinical cell therapy for PD. Stem Cells Translational Medicine 2017;6:1803-1814.
Keywords: Differentiation; Floor-plate; Midbrain dopaminergic neurons; Parkinson's disease; Transplantation; Tyrosine hydroxylase.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

Similar articles
-
Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease.Nature. 2011 Nov 6;480(7378):547-51. doi: 10.1038/nature10648. Nature. 2011. PMID: 22056989 Free PMC article.
-
Transcriptome analysis reveals transmembrane targets on transplantable midbrain dopamine progenitors.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):E1946-55. doi: 10.1073/pnas.1501989112. Epub 2015 Mar 9. Proc Natl Acad Sci U S A. 2015. PMID: 25775569 Free PMC article.
-
BMP/SMAD Pathway Promotes Neurogenesis of Midbrain Dopaminergic Neurons In Vivo and in Human Induced Pluripotent and Neural Stem Cells.J Neurosci. 2018 Feb 14;38(7):1662-1676. doi: 10.1523/JNEUROSCI.1540-17.2018. Epub 2018 Jan 10. J Neurosci. 2018. PMID: 29321139 Free PMC article.
-
Development and Differentiation of Midbrain Dopaminergic Neuron: From Bench to Bedside.Cells. 2020 Jun 18;9(6):1489. doi: 10.3390/cells9061489. Cells. 2020. PMID: 32570916 Free PMC article. Review.
-
Progress in Dopaminergic Cell Replacement and Regenerative Strategies for Parkinson's Disease.ACS Chem Neurosci. 2019 Feb 20;10(2):839-851. doi: 10.1021/acschemneuro.8b00389. Epub 2018 Oct 24. ACS Chem Neurosci. 2019. PMID: 30346716 Review.
Cited by
-
In Vivo Phenotyping of Familial Parkinson's Disease with Human Induced Pluripotent Stem Cells: A Proof-of-Concept Study.Neurochem Res. 2019 Jun;44(6):1475-1493. doi: 10.1007/s11064-019-02781-w. Epub 2019 Apr 15. Neurochem Res. 2019. PMID: 30989481
-
Effects of Passage Number and Differentiation Protocol on the Generation of Dopaminergic Neurons from Rat Bone Marrow-Derived Mesenchymal Stem Cells.Int J Mol Sci. 2018 Mar 2;19(3):720. doi: 10.3390/ijms19030720. Int J Mol Sci. 2018. PMID: 29498713 Free PMC article.
-
Emulating Human Tissues and Organs: A Bioprinting Perspective Toward Personalized Medicine.Chem Rev. 2020 Oct 14;120(19):11128-11174. doi: 10.1021/acs.chemrev.0c00342. Epub 2020 Sep 16. Chem Rev. 2020. PMID: 32937071 Free PMC article. Review.
-
Long-Term Evaluation of Intranigral Transplantation of Human iPSC-Derived Dopamine Neurons in a Parkinson's Disease Mouse Model.Cells. 2022 May 10;11(10):1596. doi: 10.3390/cells11101596. Cells. 2022. PMID: 35626637 Free PMC article.
-
Dopamine transporter neuroimaging accurately assesses the maturation of dopamine neurons in a preclinical model of Parkinson's disease.Stem Cell Res Ther. 2020 Aug 8;11(1):347. doi: 10.1186/s13287-020-01868-4. Stem Cell Res Ther. 2020. PMID: 32771055 Free PMC article.
References
-
- Nishimura K, Takahashi J. Therapeutic application of stem cell technology toward the treatment of Parkinson's disease. Biol Pharm Bull 2013;36:171–175. - PubMed
-
- Bega D, Krainc D. Long‐term clinical outcomes after fetal cell transplantation in Parkinson disease: Implications for the future of cell therapy. JAMA 2014;311:617–618. - PubMed
-
- Lindvall O, Brundin P, Widner H et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science 1990;247:574–577. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

