Defective glucocorticoid receptor signaling and keratinocyte-autonomous defects contribute to skin phenotype of mouse embryos lacking the Hsp90 co-chaperone p23
- PMID: 28650975
- PMCID: PMC5484504
- DOI: 10.1371/journal.pone.0180035
Defective glucocorticoid receptor signaling and keratinocyte-autonomous defects contribute to skin phenotype of mouse embryos lacking the Hsp90 co-chaperone p23
Abstract
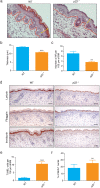
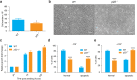
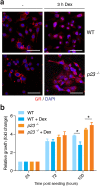
p23 is a small acidic protein with intrinsic molecular chaperone activity. It is best known as a co-chaperone of the major cytosolic molecular chaperone Hsp90. p23 binds the N-terminus of Hsp90 and stabilizes the ATP-bound and N-terminally closed Hsp90 dimer. It is in this configuration that many Hsp90 clients are most stably bound. Considering the important role of p23 in the Hsp90 cycle, it came as a surprise that it is not absolutely essential for viability in the budding yeast or for mouse development. Mice without p23 develop quite normally until birth and then all die perinatally because of immature lungs. The only other apparent phenotype of late stage embryos and newborns is a skin defect, which we have further characterized here. We found that skin differentiation is impaired, and that both apoptosis and cell proliferation are augmented in the absence of p23; the consequences are a severe thinning of the stratum corneum and reduced numbers of hair follicles. The altered differentiation, spontaneous apoptosis and proliferation are all mimicked by isolated primary keratinocytes indicating that they do require p23 functions in a cell-autonomous fashion. Since the phenotype of p23-null embryos is strikingly similar to that of embryos lacking the glucocorticoid receptor, a paradigmatic Hsp90-p23 client protein, we investigated glucocorticoid signaling. We discovered that it is impaired in vivo and for some aspects in isolated keratinocytes. Our results suggest that part of the phenotype of p23-null embryos can be explained by an impact on this particular Hsp90 client, but do not exclude that p23 by itself or in association with Hsp90 affects skin development and homeostasis through yet other pathways.
Conflict of interest statement
Figures



References
-
- Johnson JL, Toft DO. A novel chaperone complex for steroid receptors involving heat shock proteins, immunophilins, and p23. J Biol Chem. 1994;269: 24989–24993. - PubMed
-
- Dittmar KD, Hutchison KA, Owens-Grillo JK, Pratt WB. Reconstitution of the steroid receptor.hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem. 1996;271: 12833–12839. - PubMed
-
- Prodromou C, Panaretou B, Chohan S, Siligardi G, O'Brien R, Ladbury JE, et al. The ATPase cycle of Hsp90 drives a molecular 'clamp' via transient dimerization of the N-terminal domains. EMBO J. 2000;19: 4383–4392. doi: 10.1093/emboj/19.16.4383 - DOI - PMC - PubMed
-
- Siligardi G, Hu B, Panaretou B, Piper PW, Pearl LH, Prodromou C. Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J Biol Chem. 2004;279: 51989–51998. doi: 10.1074/jbc.M410562200 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

