Macrophages, but not neutrophils, are critical for proliferation of Burkholderia cenocepacia and ensuing host-damaging inflammation
- PMID: 28651010
- PMCID: PMC5501683
- DOI: 10.1371/journal.ppat.1006437
Macrophages, but not neutrophils, are critical for proliferation of Burkholderia cenocepacia and ensuing host-damaging inflammation
Erratum in
-
Correction: Macrophages, but not neutrophils, are critical for proliferation of Burkholderia cenocepacia and ensuing host-damaging inflammation.PLoS Pathog. 2017 Dec 20;13(12):e1006795. doi: 10.1371/journal.ppat.1006795. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29261811 Free PMC article.
Abstract
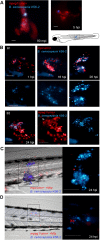
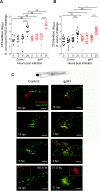
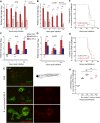
Bacteria of the Burkholderia cepacia complex (Bcc) can cause devastating pulmonary infections in cystic fibrosis (CF) patients, yet the precise mechanisms underlying inflammation, recurrent exacerbations and transition from chronic stages to acute infection and septicemia are not known. Bcc bacteria are generally believed to have a predominant extracellular biofilm life style in infected CF lungs, similar to Pseudomonas aeruginosa, but this has been challenged by clinical observations which show Bcc bacteria predominantly in macrophages. More recently, Bcc bacteria have emerged in nosocomial infections of patients hospitalized for reasons unrelated to CF. Research has abundantly shown that Bcc bacteria can survive and replicate in mammalian cells in vitro, yet the importance of an intracellular life style during infection in humans is unknown. Here we studied the contribution of innate immune cell types to fatal pro-inflammatory infection caused by B. cenocepacia using zebrafish larvae. In strong contrast to the usual protective role for macrophages against microbes, our results show that these phagocytes significantly worsen disease outcome. We provide new insight that macrophages are critical for multiplication of B. cenocepacia in the host and for development of a fatal, pro-inflammatory response that partially depends on Il1-signalling. In contrast, neutrophils did not significantly contribute to disease outcome. In subcutaneous infections that are dominated by neutrophil-driven phagocytosis, the absence of a functional NADPH oxidase complex resulted in a small but measurably higher increase in bacterial growth suggesting the oxidative burst helps limit bacterial multiplication; however, neutrophils were unable to clear the bacteria. We suggest that paradigm-changing approaches are needed for development of novel antimicrobials to efficiently disarm intracellular bacteria of this group of highly persistent, opportunistic pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
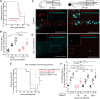
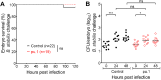
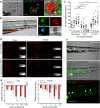
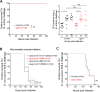


Comment in
-
Macrophages as drivers of an opportunistic infection.Microb Cell. 2017 Sep 13;4(10):362-364. doi: 10.15698/mic2017.10.595. Microb Cell. 2017. PMID: 29082233 Free PMC article.
References
-
- Drevinek P, Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect. 2010; 16(7):821–30. doi: 10.1111/j.1469-0691.2010.03237.x - DOI - PubMed
-
- Medina-Pascual M, Valdezate S, Villalón P, Garrido N, Rubio V, Saéz-Nieto J. Identification, molecular characterisation and antimicrobial susceptibility of genomovars of the Burkholderia cepacia complex in Spain. Eur J Clin Microbiol Infect Dis. 2012;31:3385–96. doi: 10.1007/s10096-012-1707-6 - DOI - PubMed
-
- Bressler A, Kaye K, LiPuma J, Alexander B, Moore C, Reller L, et al. Risk factors for Burkholderia cepacia complex bacteremia among intensive care unit patients without Cystic Fibrosis: A case-control study. Infect Control Hosp Epidemiol. 2007;28(8):951–8. doi: 10.1086/519177 - DOI - PubMed
-
- Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, et al. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros. 2012; 11(5):363–82. doi: 10.1016/j.jcf.2012.07.003 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

