Detection of circulating tumor cells from cryopreserved human sarcoma peripheral blood mononuclear cells
- PMID: 28652021
- PMCID: PMC5546157
- DOI: 10.1016/j.canlet.2017.05.032
Detection of circulating tumor cells from cryopreserved human sarcoma peripheral blood mononuclear cells
Abstract
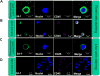
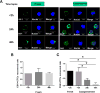
Circulating tumor cells (CTCs) enter the vasculature or lymphatic system after shedding from the primary tumor. CTCs may serve as "seed" cells for tumor metastasis. The utility of CTCs in clinical applications for sarcoma is not fully investigated, partly owing to the necessity for fresh blood samples and the lack of a CTC-specific antibody. To overcome these drawbacks, we developed a technique for sarcoma CTCs capture and detection using cryopreserved peripheral blood mononuclear cells (PBMCs) and our proprietary cell-surface vimentin (CSV) antibody 84-1, which is specific to tumor cells. This technique was validated by sarcoma cell spiking assay, matched CTCs comparison between fresh and cryopreserved PBMCs, and independent tumor markers in multiple types of sarcoma patient blood samples. The reproducibility was maximized when cryopreserved PBMCs were prepared from fresh blood samples within 2 h of the blood draw. In summary, as far as we are aware, ours is the first report to capture and detect CTCs from cryopreserved PBMCs. Further validation in other types of tumor may help boost the feasibility and utility of CTC-based diagnosis in a centralized laboratory.
Keywords: Cell surface vimentin; Circulating tumor cells; Cryopreservation; Peripheral blood mononuclear cells; Sarcoma.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Cryopreservation for delayed circulating tumor cell isolation is a valid strategy for prognostic association of circulating tumor cells in gastroesophageal cancer.World J Gastroenterol. 2018 Feb 21;24(7):810-818. doi: 10.3748/wjg.v24.i7.810. World J Gastroenterol. 2018. PMID: 29467551 Free PMC article.
-
Detection of tumor-associated cells in cryopreserved peripheral blood mononuclear cell samples for retrospective analysis.J Transl Med. 2016 Jul 2;14(1):198. doi: 10.1186/s12967-016-0953-2. J Transl Med. 2016. PMID: 27369977 Free PMC article.
-
Cell-Free RNA Content in Peripheral Blood as Potential Biomarkers for Detecting Circulating Tumor Cells in Non-Small Cell Lung Carcinoma.Int J Mol Sci. 2016 Nov 5;17(11):1845. doi: 10.3390/ijms17111845. Int J Mol Sci. 2016. PMID: 27827952 Free PMC article.
-
Circulating tumor cells in sarcomas: a brief review.Med Oncol. 2015 Jan;32(1):430. doi: 10.1007/s12032-014-0430-9. Epub 2014 Dec 10. Med Oncol. 2015. PMID: 25491143 Review.
-
[Identification of circulating tumor cells as a promising method of genitourinary cancer diagnosis].Postepy Hig Med Dosw (Online). 2012 Dec 7;66:983-90. doi: 10.5604/17322693.1023087. Postepy Hig Med Dosw (Online). 2012. PMID: 23687217 Review. Polish.
Cited by
-
Purification of viable peripheral blood mononuclear cells for biobanking using a robotized liquid handling workstation.J Transl Med. 2019 Nov 12;17(1):371. doi: 10.1186/s12967-019-2125-7. J Transl Med. 2019. PMID: 31718655 Free PMC article.
-
Multifaceted Approaches in Epithelial Cell Adhesion Molecule-Mediated Circulating Tumor Cell Isolation.Molecules. 2025 Feb 20;30(5):976. doi: 10.3390/molecules30050976. Molecules. 2025. PMID: 40076201 Free PMC article. Review.
-
Development of Surgical and Visualization Procedures to Analyze Vasculatures by Mouse Tail Edema Model.Biol Proced Online. 2021 Nov 11;23(1):21. doi: 10.1186/s12575-021-00159-3. Biol Proced Online. 2021. PMID: 34758723 Free PMC article.
-
Discovery of Cell-Surface Vimentin (CSV) as a Sarcoma Target and Development of CSV-Targeted IL12 Immune Therapy.Adv Exp Med Biol. 2020;1257:169-178. doi: 10.1007/978-3-030-43032-0_14. Adv Exp Med Biol. 2020. PMID: 32483739 Review.
-
Sarcoma treatment in the era of molecular medicine.EMBO Mol Med. 2020 Nov 6;12(11):e11131. doi: 10.15252/emmm.201911131. Epub 2020 Oct 13. EMBO Mol Med. 2020. PMID: 33047515 Free PMC article. Review.
References
-
- Mackall CL, Meltzer PS, Helman LJ. Focus on sarcomas. Cancer cell. 2002;2:175–178. - PubMed
-
- Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clinical chemistry. 2013;59:110–118. - PubMed
-
- Pantel K, Speicher M. The biology of circulating tumor cells. Oncogene. 2016;35:1216–1224. - PubMed
-
- Urtishak S, Alpaugh R, Weiner L, Swaby R. Clinical utility of circulating tumor cells: a role for monitoring response to therapy and drug development. Biomarkers in medicine. 2008;2:137–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

