Preclinical Characterization of PC786, an Inhaled Small-Molecule Respiratory Syncytial Virus L Protein Polymerase Inhibitor
- PMID: 28652242
- PMCID: PMC5571287
- DOI: 10.1128/AAC.00737-17
Preclinical Characterization of PC786, an Inhaled Small-Molecule Respiratory Syncytial Virus L Protein Polymerase Inhibitor
Abstract
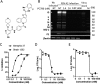
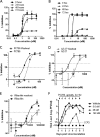
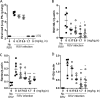
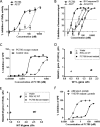
Although respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infection in infants and young children, attempts to develop an effective therapy have so far proved unsuccessful. Here we report the preclinical profiles of PC786, a potent nonnucleoside RSV L protein polymerase inhibitor, designed for inhalation treatment of RSV infection. PC786 demonstrated a potent and selective antiviral activity against laboratory-adapted or clinical isolates of RSV-A (50% inhibitory concentration [IC50], <0.09 to 0.71 nM) and RSV-B (IC50, 1.3 to 50.6 nM), which were determined by inhibition of cytopathic effects in HEp-2 cells without causing detectable cytotoxicity. The underlying inhibition of virus replication was confirmed by PCR analysis. The effects of PC786 were largely unaffected by the multiplicity of infection (MOI) and were retained in the face of established RSV replication in a time-of-addition study. Persistent anti-RSV effects of PC786 were also demonstrated in human bronchial epithelial cells. In vivo intranasal once daily dosing with PC786 was able to reduce the virus load to undetectable levels in lung homogenates from RSV-infected mice and cotton rats. Treatment with escalating concentrations identified a dominant mutation in the L protein (Y1631H) in vitro In addition, PC786 potently inhibited RSV RNA-dependent RNA polymerase (RdRp) activity in a cell-free enzyme assay and minigenome assay in HEp-2 cells (IC50, 2.1 and 0.5 nM, respectively). Thus, PC786 was shown to be a potent anti-RSV agent via inhibition of RdRp activity, making topical treatment with this compound a novel potential therapy for the treatment of human RSV infections.
Keywords: L protein; PC786; RNA polymerases; bronchial epithelial cell; clinical isolate; inhalation; polymerase; respiratory syncytial virus.
Copyright © 2017 Coates et al.
Figures
Comment in
-
The Imperative Authentication of Cell Lines.Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01823-17. doi: 10.1128/AAC.01823-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 29066454 Free PMC article. No abstract available.
-
Reply to Rojas, "The Imperative Authentication of Cell Lines".Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01827-17. doi: 10.1128/AAC.01827-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 29066455 Free PMC article. No abstract available.
Similar articles
-
Differential antiviral activities of respiratory syncytial virus (RSV) inhibitors in human airway epithelium.J Antimicrob Chemother. 2018 Jul 1;73(7):1823-1829. doi: 10.1093/jac/dky089. J Antimicrob Chemother. 2018. PMID: 29596680 Free PMC article.
-
Late therapeutic intervention with a respiratory syncytial virus L-protein polymerase inhibitor, PC786, on respiratory syncytial virus infection in human airway epithelium.Br J Pharmacol. 2018 Jun;175(12):2520-2534. doi: 10.1111/bph.14221. Epub 2018 May 2. Br J Pharmacol. 2018. PMID: 29579332 Free PMC article.
-
Interaction Between the Matrix Protein and the Polymerase Complex of Respiratory Syncytial Virus.Viruses. 2024 Dec 4;16(12):1881. doi: 10.3390/v16121881. Viruses. 2024. PMID: 39772190 Free PMC article.
-
New antiviral approaches for respiratory syncytial virus and other mononegaviruses: Inhibiting the RNA polymerase.Antiviral Res. 2016 Oct;134:63-76. doi: 10.1016/j.antiviral.2016.08.006. Epub 2016 Aug 27. Antiviral Res. 2016. PMID: 27575793 Review.
-
Novel therapies for an old virus: treatment of RSV infections in the 21st Century.Expert Rev Anti Infect Ther. 2009 Nov;7(9):1125-9. doi: 10.1586/eri.09.90. Expert Rev Anti Infect Ther. 2009. PMID: 19883332 Review.
Cited by
-
Analysis of Template Variations on RNA Synthesis by Respiratory Syncytial Virus Polymerase.Viruses. 2022 Dec 23;15(1):47. doi: 10.3390/v15010047. Viruses. 2022. PMID: 36680087 Free PMC article.
-
Differential antiviral activities of respiratory syncytial virus (RSV) inhibitors in human airway epithelium.J Antimicrob Chemother. 2018 Jul 1;73(7):1823-1829. doi: 10.1093/jac/dky089. J Antimicrob Chemother. 2018. PMID: 29596680 Free PMC article.
-
Structural basis of paramyxo- and pneumovirus polymerase inhibition by non-nucleoside small-molecule antivirals.Antimicrob Agents Chemother. 2024 Oct 8;68(10):e0080024. doi: 10.1128/aac.00800-24. Epub 2024 Aug 20. Antimicrob Agents Chemother. 2024. PMID: 39162479 Free PMC article. Review.
-
Conformationally restricted benzothienoazepine respiratory syncytial virus inhibitors: their synthesis, structural analysis and biological activities.Medchemcomm. 2018 Feb 20;9(3):583-589. doi: 10.1039/c8md00033f. eCollection 2018 Mar 1. Medchemcomm. 2018. PMID: 30108949 Free PMC article.
-
Prevention and Potential Treatment Strategies for Respiratory Syncytial Virus.Molecules. 2024 Jan 25;29(3):598. doi: 10.3390/molecules29030598. Molecules. 2024. PMID: 38338343 Free PMC article. Review.
References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

