Posttranscriptional Upregulation of IDH1 by HuR Establishes a Powerful Survival Phenotype in Pancreatic Cancer Cells
- PMID: 28652247
- PMCID: PMC5922269
- DOI: 10.1158/0008-5472.CAN-17-0015
Posttranscriptional Upregulation of IDH1 by HuR Establishes a Powerful Survival Phenotype in Pancreatic Cancer Cells
Abstract
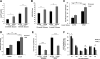
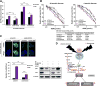
Cancer aggressiveness may result from the selective pressure of a harsh nutrient-deprived microenvironment. Here we illustrate how such conditions promote chemotherapy resistance in pancreatic ductal adenocarcinoma (PDAC). Glucose or glutamine withdrawal resulted in a 5- to 10-fold protective effect with chemotherapy treatment. PDAC xenografts were less sensitive to gemcitabine in hypoglycemic mice compared with hyperglycemic mice. Consistent with this observation, patients receiving adjuvant gemcitabine (n = 107) with elevated serum glucose levels (HgbA1C > 6.5%) exhibited improved survival. We identified enhanced antioxidant defense as a driver of chemoresistance in this setting. ROS levels were doubled in vitro by either nutrient withdrawal or gemcitabine treatment, but depriving PDAC cells of nutrients before gemcitabine treatment attenuated this effect. Mechanistic investigations based on RNAi or CRISPR approaches implicated the RNA binding protein HuR in preserving survival under nutrient withdrawal, with or without gemcitabine. Notably, RNA deep sequencing and functional analyses in HuR-deficient PDAC cell lines identified isocitrate dehydrogenase 1 (IDH1) as the sole antioxidant enzyme under HuR regulation. HuR-deficient PDAC cells lacked the ability to engraft successfully in immunocompromised mice, but IDH1 overexpression in these cells was sufficient to fully restore chemoresistance under low nutrient conditions. Overall, our findings highlight the HuR-IDH1 regulatory axis as a critical, actionable therapeutic target in pancreatic cancer. Cancer Res; 77(16); 4460-71. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
C.M. Metallo is a consultant/advisory board member of Agios Pharmaceuticals. No potential conflicts of interest were disclosed by the other authors.
Figures

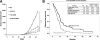



References
-
- Ros PR, Mortele KJ. Imaging features of pancreatic neoplasms. JBR-BTR. 2001;84:239–49. - PubMed
-
- Morgan G, Ward R, Barton M. The contribution of cytotoxic chemotherapy to 5-year survival in adult malignancies. Clin Oncol. 2004;16:549–60. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

