Metabolism Dealing with Thermal Degradation of NAD+ in the Hyperthermophilic Archaeon Thermococcus kodakarensis
- PMID: 28652302
- PMCID: PMC5585711
- DOI: 10.1128/JB.00162-17
Metabolism Dealing with Thermal Degradation of NAD+ in the Hyperthermophilic Archaeon Thermococcus kodakarensis
Abstract
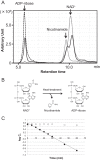
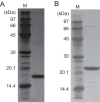
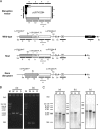
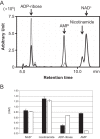
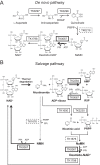
NAD+ is an important cofactor for enzymatic oxidation reactions in all living organisms, including (hyper)thermophiles. However, NAD+ is susceptible to thermal degradation at high temperatures. It can thus be expected that (hyper)thermophiles harbor mechanisms that maintain in vivo NAD+ concentrations and possibly remove and/or reuse undesirable degradation products of NAD+ Here we confirmed that at 85°C, thermal degradation of NAD+ results mostly in the generation of nicotinamide and ADP-ribose, the latter known to display toxicity by spontaneously linking to proteins. The hyperthermophilic archaeon Thermococcus kodakarensis possesses a putative ADP-ribose pyrophosphatase (ADPR-PPase) encoded by the TK2284 gene. ADPR-PPase hydrolyzes ADP-ribose to ribose 5-phosphate (R5P) and AMP. The purified recombinant TK2284 protein exhibited activity toward ADP-ribose as well as ADP-glucose. Kinetic analyses revealed a much higher catalytic efficiency toward ADP-ribose, suggesting that ADP-ribose was the physiological substrate. To gain insight into the physiological function of TK2284, a TK2284 gene disruption strain was constructed and examined. Incubation of NAD+ in the cell extract of the mutant strain at 85°C resulted in higher ADP-ribose accumulation and lower AMP production compared with those in experiments with the host strain cell extract. The mutant strain also exhibited lower cell yield and specific growth rates in a synthetic amino acid medium compared with those of the host strain. The results obtained here suggest that the ADPR-PPase in T. kodakarensis is responsible for the cleavage of ADP-ribose to R5P and AMP, providing a means to utilize the otherwise dead-end product of NAD+ breakdown.IMPORTANCE Hyperthermophilic microorganisms living under high temperature conditions should have mechanisms that deal with the degradation of thermolabile molecules. NAD+ is an important cofactor for enzymatic oxidation reactions and is susceptible to thermal degradation to ADP-ribose and nicotinamide. Here we show that an ADP-ribose pyrophosphatase homolog from the hyperthermophilic archaeon Thermococcus kodakarensis converts the detrimental ADP-ribose to ribose 5-phosphate and AMP, compounds that can be directed to central carbon metabolism. This physiological role for ADP-ribose pyrophosphatases might be universal in hyperthermophiles, as their homologs are widely distributed among both hyperthermophilic bacteria and archaea.
Keywords: ADP-ribose pyrophosphatase; Archaea; NAD+; Thermococcus; hyperthermophiles; thermal degradation.
Copyright © 2017 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

