Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics
- PMID: 28652357
- PMCID: PMC5514729
- DOI: 10.1073/pnas.1702275114
Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics
Abstract
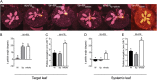
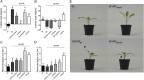
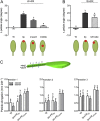
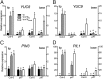
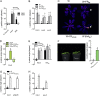
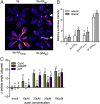
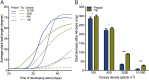
Vegetation stands have a heterogeneous distribution of light quality, including the red/far-red light ratio (R/FR) that informs plants about proximity of neighbors. Adequate responses to changes in R/FR are important for competitive success. How the detection and response to R/FR are spatially linked and how this spatial coordination between detection and response affects plant performance remains unresolved. We show in Arabidopsis thaliana and Brassica nigra that localized FR enrichment at the lamina tip induces upward leaf movement (hyponasty) from the petiole base. Using a combination of organ-level transcriptome analysis, molecular reporters, and physiology, we show that PIF-dependent spatial auxin dynamics are key to this remote response to localized FR enrichment. Using computational 3D modeling, we show that remote signaling of R/FR for hyponasty has an adaptive advantage over local signaling in the petiole, because it optimizes the timing of leaf movement in response to neighbors and prevents hyponasty caused by self-shading.
Keywords: auxin; functional-structural plant model; leaf movement; phytochrome; shade avoidance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Local auxin production underlies a spatially restricted neighbor-detection response in Arabidopsis.Proc Natl Acad Sci U S A. 2017 Jul 11;114(28):7444-7449. doi: 10.1073/pnas.1702276114. Epub 2017 Jun 26. Proc Natl Acad Sci U S A. 2017. PMID: 28652343 Free PMC article.
-
Local light signaling at the leaf tip drives remote differential petiole growth through auxin-gibberellin dynamics.Curr Biol. 2023 Jan 9;33(1):75-85.e5. doi: 10.1016/j.cub.2022.11.045. Epub 2022 Dec 19. Curr Biol. 2023. PMID: 36538931 Free PMC article.
-
Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and della proteins in Arabidopsis.Plant Physiol. 2009 Apr;149(4):1701-12. doi: 10.1104/pp.108.133496. Epub 2009 Feb 11. Plant Physiol. 2009. PMID: 19211699 Free PMC article.
-
Multiple links between shade avoidance and auxin networks.J Exp Bot. 2018 Jan 4;69(2):213-228. doi: 10.1093/jxb/erx295. J Exp Bot. 2018. PMID: 29036463 Review.
-
Hormonal Regulation in Shade Avoidance.Front Plant Sci. 2017 Sep 4;8:1527. doi: 10.3389/fpls.2017.01527. eCollection 2017. Front Plant Sci. 2017. PMID: 28928761 Free PMC article. Review.
Cited by
-
Shade suppresses wound-induced leaf repositioning through a mechanism involving PHYTOCHROME KINASE SUBSTRATE (PKS) genes.PLoS Genet. 2022 May 27;18(5):e1010213. doi: 10.1371/journal.pgen.1010213. eCollection 2022 May. PLoS Genet. 2022. PMID: 35622862 Free PMC article.
-
Species-specific PHYTOCHROME-INTERACTING FACTOR utilization in the plant morphogenetic response to environmental stimuli.Plant Cell. 2025 May 9;37(5):koaf048. doi: 10.1093/plcell/koaf048. Plant Cell. 2025. PMID: 40085779 Free PMC article.
-
Reducing shade avoidance can improve Arabidopsis canopy performance against competitors.Plant Cell Environ. 2021 Apr;44(4):1130-1141. doi: 10.1111/pce.13905. Epub 2020 Oct 19. Plant Cell Environ. 2021. PMID: 33034378 Free PMC article.
-
Molecular mechanisms underlying phytochrome-controlled morphogenesis in plants.Nat Commun. 2019 Nov 19;10(1):5219. doi: 10.1038/s41467-019-13045-0. Nat Commun. 2019. PMID: 31745087 Free PMC article. Review.
-
Spatial regulation of plant hormone action.J Exp Bot. 2023 Oct 13;74(19):6089-6103. doi: 10.1093/jxb/erad244. J Exp Bot. 2023. PMID: 37401809 Free PMC article. Review.
References
-
- Crepy MA, Casal JJ. Photoreceptor-mediated kin recognition in plants. New Phytol. 2015;205:329–338. - PubMed
-
- Chelle M, et al. Simulation of the three-dimensional distribution of the red:far-red ratio within crop canopies. New Phytol. 2007;176:223–234. - PubMed
-
- Ballaré CL, Sanchez RA, Scopel AL, Casal JJ, Ghersa CM. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ. 1987;10:551–557.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

