Medication-related issues associated with adherence to long-term tyrosine kinase inhibitors for controlling chronic myeloid leukemia: a qualitative study
- PMID: 28652712
- PMCID: PMC5476765
- DOI: 10.2147/PPA.S132894
Medication-related issues associated with adherence to long-term tyrosine kinase inhibitors for controlling chronic myeloid leukemia: a qualitative study
Abstract
Purpose: Poor adherence to tyrosine kinase inhibitors (TKIs) could compromise the control of chronic myeloid leukemia (CML) and contributes to poorer survival. Little is known about how medication-related issues affect CML patients' adherence to TKI therapy in Malaysia. This qualitative study aimed to explore these issues.
Patients and methods: Individual face-to-face, semistructured interviews were conducted at the hematology outpatient clinics of two medical centers in Malaysia from August 2015 to January 2016. CML patients aged ≥18 years who were prescribed a TKI were invited to participate in the study. Interviews were audio-recorded, transcribed verbatim, and thematically analyzed.
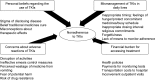
Results: Four themes were identified from 18 interviews: 1) concerns about adverse reactions to TKIs, 2) personal beliefs regarding the use of TKIs, 3) mismanagement of TKIs in daily lives, and 4) financial burden in accessing treatment. Participants skipped their TKIs due to ineffective emesis control measures and perceived wastage of medication from vomiting. Participants also modified their TKI therapy due to fear of potential harm from long-term use, and stopped taking their TKIs based on belief in curative claims of traditional medicines and misconception about therapeutic effects of TKIs. Difficulty in integrating the dosing requirements of TKIs into daily lives led to unintentional skipping of doses, as well as the risk of toxicities from inappropriate dosing intervals or food interactions. Furthermore, financial constraints also resulted in delayed initiation of TKIs, missed clinic appointments, and treatment interruptions.
Conclusion: Malaysian CML patients encountered a range of medication-related issues leading to a complex pattern of nonadherence to TKI therapy. Further studies should investigate whether regular contact with patients to improve understanding of treatment rationale, to elicit and address patients' concerns about adverse reactions, and to empower patients with skills to self-manage their medications might promote better adherence to TKIs and improve CML patients' outcome.
Keywords: adherence; chronic myeloid leukemia; medication-related issues; qualitative study; thematic analysis; tyrosine kinase inhibitors.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Hoglund M, Sandin F, Simonsson B. Epidemiology of chronic myeloid leukaemia: an update. Ann Hematol. 2015;94(Suppl 2):S241–S247. - PubMed
-
- Omar ZA, Ibrahim Tamin NS. National Cancer Registry Report 2007. Kuala Lumpur: Ministry of Health Malaysia; 2011.
-
- Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. - PubMed
-
- Ross DM, Branford S, Seymour JF, et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122(4):515–522. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources