Can we improve pain and sleep in elderly individuals with transcranial direct current stimulation? - Results from a randomized controlled pilot study
- PMID: 28652716
- PMCID: PMC5472413
- DOI: 10.2147/CIA.S133423
Can we improve pain and sleep in elderly individuals with transcranial direct current stimulation? - Results from a randomized controlled pilot study
Abstract
Background: The prevalence of chronic pain and sleep disturbances substantially increases with age. Pharmacotherapy remains the primary treatment option for these health issues. However, side effects and drug interactions are difficult to control in elderly individuals.
Aims: The objective of this study was to assess the feasibility of conducting a randomized sham-controlled trial and to collect preliminary data on the efficacy of transcranial direct current stimulation (tDCS) to reduce pain and improve sleep in older adults suffering from chronic pain.
Methods: Fourteen elderly individuals (mean age 71±7 years) suffering from chronic pain and sleep complaints were randomized to receive either anodal tDCS, applied over the primary motor cortex (2 mA, 20 minutes), or sham tDCS, for 5 consecutive days. Pain was measured with visual analog scales, pain logbooks and questionnaires, while sleep was assessed with actigraphy, sleep diaries and questionnaires.
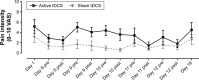
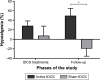
Results: There were no missing data for pain and sleep measures, except for actigraphy, that generated several missing data. Blinding was maintained throughout the study, for both the evaluator and participants. Active but not sham tDCS significantly reduced pain (P<0.05). No change was observed in sleep parameters, in both the active and sham tDCS groups (all P≥0.18).
Conclusion: The present study provides guidelines for the implementation of future tDCS studies in larger populations of elderly individuals. M1 anodal tDCS in this population appears to be effective to reduce pain, but not to improve sleep.
Keywords: actigraphy; aging; elderly; pain; sleep; tDCS; transcranial direct current stimulation.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Manocchia M, Keller S, Ware JE. Sleep problems, health-related quality of life, work functioning and health care utilization among the chronically ill. Qual Life Res. 2001;10(4):331–345. - PubMed
-
- Marchand S. Le phénomène de la douleur [The Phenomenon of Pain] Montreal: Les Éditions de la Chenelière Inc; 2009. French.
-
- Cho CH, Jung SW, Park JY, Song KS, Yu KI. Is shoulder pain for three months or longer correlated with depression, anxiety, and sleep disturbance? J Shoulder Elbow Surg. 2013;22(2):222–228. - PubMed
-
- Fishbain DA, Cole B, Lewis JE, Gao J. What is the evidence for chronic pain being etiologically associated with the DSM-IV category of sleep disorder due to a general medical condition? A structured evidence-based review. Pain Med. 2010;11(2):158–179. - PubMed
-
- Onen SH, Onen F, Courpron P, Dubray C. How pain and analgesics disturb sleep. Clin J Pain. 2005;21(5):422–431. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

